RNA-binding protein YBX3 promotes PPARγ-SLC3A2 mediated BCAA metabolism fueling brown adipogenesis and thermogenesis
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objective
Activating brown adipose tissue (BAT) thermogenesis is a promising approach to combat obesity and metabolic disorders. The post-transcriptional regulation of BAT thermogenesis mediated by RNA-binding proteins (RBPs) is still not fully understood. This study explores the physiological role of novel RBPs in BAT differentiation and thermogenesis.
Methods
We used multiple public datasets to screen out novel RBPs responsible for BAT differentiation and thermogenesis. In vitro loss- and gain-of-function experiments were performed in both C3H10T1/2 preadipocytes and mature brown adipocytes to determine the role of Y-box binding protein 3 (YBX3) in brown adipocyte differentiation and thermogenesis. Adeno-associated virus (AAV)-mediated BAT-specific knockdown or overexpression of Ybx3 was applied to investigate the function of YBX3 in vivo.
Results
YBX3 is a brown adipocyte-enriched RBP induced by cold stimulation and β-adrenergic signaling. Both in vitro loss- and gain-of-function experiments demonstrate that YBX3 is essential for brown adipocyte differentiation and thermogenesis. BAT-specific loss of Ybx3 dampens thermogenesis and exacerbates diet-induced obesity in mice, while overexpression of Ybx3 promotes thermogenesis and confers protection against diet-induced metabolic dysfunction. Transcriptome analysis and mitochondrial stress test indicate that Ybx3 deficiency compromises the mitochondrial oxidative phosphorylation, leading to thermogenic failure. Mechanistically, YBX3 stabilizes the mRNA of Slc3a2 and Pparg, which facilitates branched-chain amino acid (BCAA) influx and catabolism and fuels brown adipocyte differentiation and thermogenesis.
Conclusions
YBX3 facilitates BAT fueling BCAA to boost thermogenesis and energy expenditure, which protects against obesity and metabolic dysfunction. Thus, YBX3 could be a promising therapeutic target for obesity.
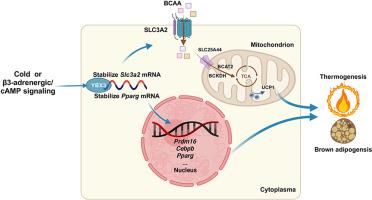
RNA结合蛋白YBX3促进PPARγ-SLC3A2介导的BCAA代谢,促进棕色脂肪生成和产热。
目的:激活棕色脂肪组织(BAT)的产热是对抗肥胖和代谢紊乱的一种很有前景的方法。由 RNA 结合蛋白(RBPs)介导的 BAT 产热的转录后调控尚未完全明了。本研究探讨了新型 RBPs 在 BAT 分化和产热过程中的生理作用:方法:我们利用多个公开数据集筛选出负责 BAT 分化和产热的新型 RBPs。我们在C3H10T1/2前脂肪细胞和成熟棕色脂肪细胞中进行了体外功能缺失和功能增益实验,以确定Y-盒结合蛋白3(YBX3)在棕色脂肪细胞分化和产热中的作用。应用腺相关病毒(AAV)介导的BAT特异性Ybx3敲除或过表达研究了YBX3在体内的功能:结果:YBX3是一种富含棕色脂肪细胞的RBP,由冷刺激和β肾上腺素能信号诱导。体外功能缺失和功能增益实验均证明,YBX3 对棕色脂肪细胞的分化和产热至关重要。BAT特异性缺失Ybx3会抑制小鼠的产热并加剧饮食诱导的肥胖,而过表达Ybx3则会促进产热并防止饮食诱导的代谢功能障碍。转录组分析和线粒体压力测试表明,Ybx3 缺乏会损害线粒体氧化磷酸化,导致产热失败。从机制上讲,YBX3能稳定Slc3a2和Pparg的mRNA,从而促进支链氨基酸(BCAA)的流入和分解,并促进棕色脂肪细胞的分化和产热:结论:YBX3 可促进 BAT 为 BCAA 提供燃料,从而促进产热和能量消耗,防止肥胖和代谢功能障碍。因此,YBX3 可作为肥胖症的治疗靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


