An optimized fractionation method reveals insulin-induced membrane surface localization of GLUT1 to increase glycolysis in LβT2 cells
IF 3.8
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Insulin is an important regulator of whole-body glucose homeostasis. In insulin sensitive tissues such as muscle and adipose, insulin induces the translocation of glucose transporter 4 (GLUT4) to the cell membrane, thereby increasing glucose uptake. However, insulin also signals in tissues that are not generally associated with glucose homeostasis. In the human reproductive endocrine axis, hyperinsulinemia suppresses the secretion of gonadotropins from gonadotrope cells of the anterior pituitary, thereby linking insulin dysregulation to suboptimal reproductive health. In the mouse, gonadotropes express the insulin receptor which has the canonical signaling response of IRS, AKT, and mTOR activation. However, the functional outcomes of insulin action on gonadotropes are unclear. Here, we demonstrate through use of an optimized cell fractionation protocol that insulin stimulation of the LβT2 gonadotropic cell line results in the unexpected translocation of GLUT1 to the plasma membrane. Using our high purity fractionation protocol, we further demonstrate that though Akt signaling in response to insulin is intact, insulin-induced translocation of GLUT1 occurs independently of Akt activation in LβT2 cells.
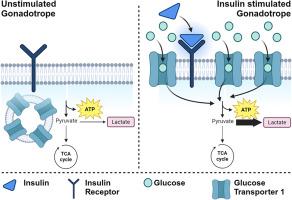
优化的分馏方法揭示了胰岛素诱导的 GLUT1 膜表面定位增加 LβT2 细胞中的糖酵解。
胰岛素是全身葡萄糖平衡的重要调节剂。在肌肉和脂肪等对胰岛素敏感的组织中,胰岛素会诱导葡萄糖转运体 4(GLUT4)转运到细胞膜上,从而增加葡萄糖的摄取。然而,胰岛素也会在通常与葡萄糖平衡无关的组织中发出信号。在人类生殖内分泌轴中,高胰岛素血症会抑制垂体前叶促性腺激素细胞分泌促性腺激素,从而将胰岛素失调与生殖健康不良联系起来。在小鼠体内,促性腺激素表达胰岛素受体,而胰岛素受体具有激活 IRS、AKT 和 mTOR 的典型信号反应。然而,胰岛素作用于促性腺激素的功能结果尚不清楚。在这里,我们通过使用优化的细胞分馏方案证明,胰岛素刺激 LβT2 促性腺激素细胞系会导致 GLUT1 意外地转位到质膜上。利用我们的高纯度分馏方案,我们进一步证明,尽管对胰岛素做出反应的 Akt 信号是完整的,但胰岛素诱导的 GLUT1 转位在 LβT2 细胞中的发生与 Akt 激活无关。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


