Aldehyde dehydrogenase-2 deficiency aggravates neuroinflammation, nociception, and motor impairment in a mouse model of multiple sclerosis
IF 7.1
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Aldehyde dehydrogenase-2 deficiency (ALDH2∗2) found in 36 % of Han Chinese, affects approximately 8 % of the world population. ALDH2 is a mitochondrial key enzyme in detoxifying reactive aldehydes to less reactive forms. Studies demonstrate a potential link between ALDH2∗2 mutation and neurodegenerative diseases. Multiple sclerosis (MS) is an incurable and disabling neurodegenerative autoimmune disease that induces motor, and cognitive impairment, and hypersensitivity, including chronic pain. Accumulating evidence suggests that reactive aldehydes, such as 4-hydroxynonenal (4-HNE), contribute to MS pathogenesis. Here, using knock-in mice carrying the inactivating point mutation in ALDH2, identical to the mutation found in Han Chinese, we showed that the impairment in ALDH2 activity heightens motor disabilities, and hypernociception induced by experimental autoimmune encephalomyelitis (EAE). The deleterious clinical signs are followed by glial cell activation in the spinal cord and increased 4-HNE levels in the spinal cord and serum. Importantly, the pharmacological ALDH2 activation by Alda-1 ameliorates EAE-induced hypernociception and motor impairment in both wild-type and ALDH2∗2KI mice. Reduced hypernociception was associated with less early growth response protein 1 (EGR1), neuronal and glial activation, and reactive aldehyde accumulation in the spinal cord and serum. Taken together, our data suggest that the mitochondrial enzyme ALDH2 plays a role in regulating clinical, cellular, and molecular responses associated with EAE. This indicates that ALDH2 could serve as a molecular target for MS control, with ALDH2 activators, like Alda-1 as potential neuroprotective candidates. Furthermore, ALDH2∗2 carriers may be at increased risk of developing more accentuated MS symptoms.
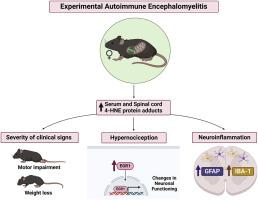
在多发性硬化症小鼠模型中,醛脱氢酶-2 缺乏会加重神经炎症、痛觉和运动障碍。
36%的汉族人患有醛脱氢酶-2缺乏症(ALDH2*2),全球约有8%的人患有这种疾病。ALDH2 是线粒体中的一种关键酶,可将活性醛解毒为活性较低的形式。研究表明,ALDH2*2 突变与神经退行性疾病之间存在潜在联系。多发性硬化症(MS)是一种无法治愈的致残性神经退行性自身免疫性疾病,会诱发运动、认知障碍和超敏反应,包括慢性疼痛。越来越多的证据表明,4-羟基壬烯醛(4-HNE)等活性醛类是多发性硬化症的发病机制之一。在此,我们利用携带 ALDH2 失活点突变(与在中国汉族发现的突变相同)的基因敲入小鼠研究发现,ALDH2 活性受损会加重运动障碍以及由实验性自身免疫性脑脊髓炎(EAE)诱发的痛觉减退。脊髓神经胶质细胞活化和脊髓及血清中4-HNE水平升高会导致有害的临床症状。重要的是,在野生型小鼠和 ALDH2*2KI 小鼠中,Alda-1 对 ALDH2 的药理激活可改善 EAE 诱导的痛觉减退和运动障碍。痛觉减退与早期生长反应蛋白 1 (EGR1)、神经元和神经胶质细胞活化以及脊髓和血清中活性醛积累减少有关。综上所述,我们的数据表明线粒体酶 ALDH2 在调节与 EAE 相关的临床、细胞和分子反应中发挥作用。这表明,ALDH2 可作为控制多发性硬化症的分子靶点,ALDH2 激活剂(如 Alda-1)可作为潜在的神经保护候选靶点。此外,ALDH2*2 携带者可能会增加出现更严重多发性硬化症症状的风险。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Free Radical Biology and Medicine
医学-内分泌学与代谢
CiteScore
14.00
自引率
4.10%
发文量
850
审稿时长
22 days
期刊介绍:
Free Radical Biology and Medicine is a leading journal in the field of redox biology, which is the study of the role of reactive oxygen species (ROS) and other oxidizing agents in biological systems. The journal serves as a premier forum for publishing innovative and groundbreaking research that explores the redox biology of health and disease, covering a wide range of topics and disciplines. Free Radical Biology and Medicine also commissions Special Issues that highlight recent advances in both basic and clinical research, with a particular emphasis on the mechanisms underlying altered metabolism and redox signaling. These Special Issues aim to provide a focused platform for the latest research in the field, fostering collaboration and knowledge exchange among researchers and clinicians.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


