SARS-CoV-2 variants mediated tissue-specific metabolic reprogramming determines the disease pathophysiology in a hamster model
IF 8.8
2区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
Despite significant effort, a clear understanding of host tissue-specific responses and their implications for immunopathogenicity against the severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) variant infection has remained poorly defined. To shed light on the interaction between tissues and SARS-CoV-2 variants, we sought to characterize the complex relationship among acute multisystem manifestations, dysbiosis of the gut microbiota, and the resulting implications for SARS-CoV-2 variant-specific immunopathogenesis in the Golden Syrian Hamster (GSH) model using multi-omics approaches. Our investigation revealed the presence of increased SARS-CoV-2 genomic RNA in diverse tissues of delta-infected GSH compared to the omicron variant. Multi-omics analyses uncovered distinctive metabolic responses between the delta and omicron variants, with the former demonstrating dysregulation in synaptic transmission proteins associated with neurocognitive disorders. Additionally, delta-infected GSH exhibited an altered fecal microbiota composition, marked by increased inflammation-associated taxa and reduced commensal bacteria compared to the omicron variant. These findings underscore the SARS-CoV-2-mediated tissue insult, characterized by modified host metabolites, neurological protein dysregulation, and gut dysbiosis, highlighting the compromised gut-lung-brain axis during acute infection.
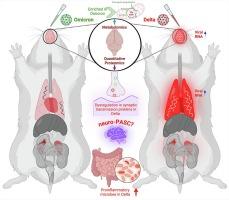
Sars-Cov-2 变体介导的组织特异性代谢组重编决定了仓鼠模型中的疾病病理生理学。
尽管付出了巨大的努力,但对宿主组织特异性反应及其对严重急性呼吸系统综合征冠状病毒2(SARS-CoV-2)变异体感染的免疫致病性的影响的清晰认识仍不十分明确。为了揭示器官与特定 SARS-CoV-2 变体之间的相互作用,我们试图利用多组学方法描述金色叙利亚仓鼠(GSH)模型中急性多系统表现、肠道微生物群失调之间的复杂关系,以及由此产生的对 SARS-CoV-2 变体特异性免疫发病机制的影响。我们的研究发现,与奥米克伦变体相比,在受delta感染的GSH的不同组织中存在更多的SARS-CoV-2基因组RNA。多组学分析发现了δ变体和Ω变体之间不同的代谢反应,前者表现出与神经认知障碍相关的突触传递蛋白失调。此外,与奥米克伦变体相比,δ感染的GSH表现出粪便微生物群组成的改变,其特点是炎症相关类群增加,共生菌减少。这些发现强调了 SARS-CoV-2 介导的组织损伤,其特点是宿主代谢物改变、神经系统蛋白质失调和肠道菌群失调,突出了急性感染期间肠道-肺-脑轴的损害。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
29.60
自引率
2.00%
发文量
290
审稿时长
28 days
期刊介绍:
Established in 1987, Brain, Behavior, and Immunity proudly serves as the official journal of the Psychoneuroimmunology Research Society (PNIRS). This pioneering journal is dedicated to publishing peer-reviewed basic, experimental, and clinical studies that explore the intricate interactions among behavioral, neural, endocrine, and immune systems in both humans and animals.
As an international and interdisciplinary platform, Brain, Behavior, and Immunity focuses on original research spanning neuroscience, immunology, integrative physiology, behavioral biology, psychiatry, psychology, and clinical medicine. The journal is inclusive of research conducted at various levels, including molecular, cellular, social, and whole organism perspectives. With a commitment to efficiency, the journal facilitates online submission and review, ensuring timely publication of experimental results. Manuscripts typically undergo peer review and are returned to authors within 30 days of submission. It's worth noting that Brain, Behavior, and Immunity, published eight times a year, does not impose submission fees or page charges, fostering an open and accessible platform for scientific discourse.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


