The Hsp90 Molecular Chaperone as a Global Modifier of the Genotype-Phenotype-Fitness Map: An Evolutionary Perspective
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Global modifier genes influence the mapping of genotypes onto phenotypes and fitness through their epistatic interactions with genetic variants on a massive scale. The first such factor to be identified, Hsp90, is a highly conserved molecular chaperone that plays a central role in protein homeostasis. Hsp90 is a “hub of hubs” that chaperones proteins engaged in many key cellular and developmental regulatory networks. These clients, which are enriched in kinases, transcription factors, and E3 ubiquitin ligases, drive diverse cellular functions and are themselves highly connected. By contrast to many other hub proteins, the abundance and activity of Hsp90 changes substantially in response to shifting environmental conditions. As a result, Hsp90 modifies the functional impact of many genetic variants simultaneously in a manner that depends on environmental stress. Studies in diverse organisms suggest that this coupling between Hsp90 function and challenging environments exerts a substantial impact on what parts of the genome are visible to natural selection, expanding adaptive opportunities when most needed. In this Perspective, we explore the multifaceted role of Hsp90 as global modifier of the genotype-phenotype-fitness map as well as its implications for evolution in nature and the clinic.
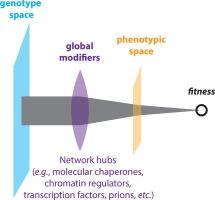
Hsp90分子伴侣是基因型-表型-适配性图谱的全球修饰因子:进化的视角
全局修饰基因通过与遗传变异的大规模表观相互作用,影响着基因型与表型的映射和适应性。第一个被发现的此类因子 Hsp90 是一种高度保守的分子伴侣,在蛋白质平衡中发挥着核心作用。Hsp90 是一个 "枢纽中的枢纽",它为参与许多关键细胞和发育调控网络的蛋白质提供伴侣。这些客户包括激酶、转录因子和 E3 泛素连接酶,它们驱动着多种细胞功能,而且本身也高度关联。与许多其他枢纽蛋白不同的是,Hsp90 的丰度和活性会随着环境条件的变化而发生重大变化。因此,Hsp90 以一种取决于环境压力的方式同时改变了许多基因变异的功能影响。对不同生物的研究表明,Hsp90 功能与挑战性环境之间的这种耦合关系对基因组中哪些部分可以被自然选择发现产生了重大影响,从而在最需要的时候扩大了适应机会。在本《视角》中,我们将探讨Hsp90作为基因型-表型-适配性图谱全球修饰因子的多方面作用及其对自然界和临床进化的影响。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


