mTORC1 activity licenses its own release from the lysosomal surface
IF 16.6
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Nutrient signaling converges on mTORC1, which, in turn, orchestrates a physiological cellular response. A key determinant of mTORC1 activity is its shuttling between the lysosomal surface and the cytoplasm, with nutrients promoting its recruitment to lysosomes by the Rag GTPases. Active mTORC1 regulates various cellular functions by phosphorylating distinct substrates at different subcellular locations. Importantly, how mTORC1 that is activated on lysosomes is released to meet its non-lysosomal targets and whether mTORC1 activity itself impacts its localization remain unclear. Here, we show that, in human cells, mTORC1 inhibition prevents its release from lysosomes, even under starvation conditions, which is accompanied by elevated and sustained phosphorylation of its lysosomal substrate TFEB. Mechanistically, “inactive” mTORC1 causes persistent Rag activation, underlining its release as another process actively mediated via the Rags. In sum, we describe a mechanism by which mTORC1 controls its own localization, likely to prevent futile cycling on and off lysosomes.
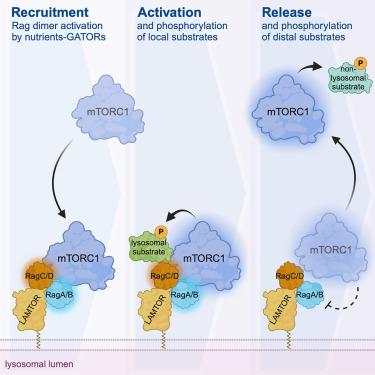
mTORC1 的活性允许自己从溶酶体表面释放出来
营养信号汇聚到 mTORC1,而 mTORC1 又反过来协调细胞的生理反应。决定 mTORC1 活性的一个关键因素是它在溶酶体表面和细胞质之间的穿梭,营养物质通过 Rag GTP 酶将其招募到溶酶体。活跃的 mTORC1 通过在不同亚细胞位置磷酸化不同的底物来调节各种细胞功能。重要的是,在溶酶体上被激活的 mTORC1 如何被释放以满足其非溶酶体目标,以及 mTORC1 活性本身是否会影响其定位,这些问题仍不清楚。在这里,我们发现,在人体细胞中,即使在饥饿条件下,抑制 mTORC1 也能阻止其从溶酶体释放,同时溶酶体底物 TFEB 的磷酸化也会升高并持续。从机理上讲,"非活性 "的 mTORC1 会导致持续的 Rag 激活,从而强调其释放是通过 Rags 积极介导的另一个过程。总之,我们描述了一种 mTORC1 控制自身定位的机制,这种机制很可能是为了防止溶酶体上和溶酶体下的徒劳循环。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


