Skeletal muscle from TBC1D4 p.Arg684Ter variant carriers is severely insulin resistant but exhibits normal metabolic responses during exercise
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
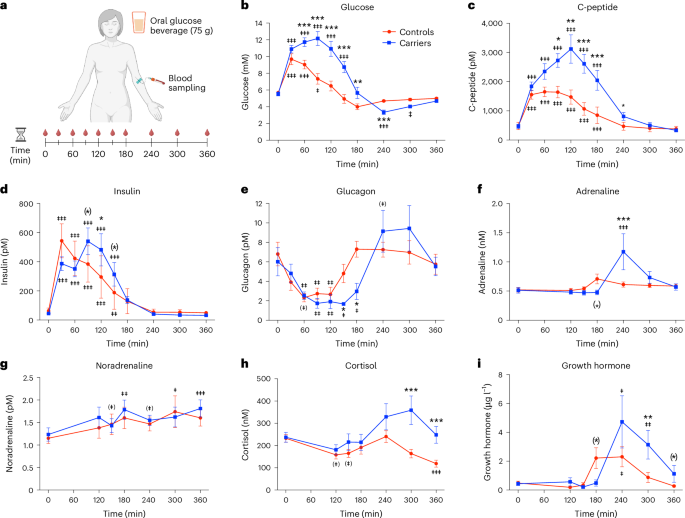
In the Greenlandic Inuit population, 4% are homozygous carriers of a genetic nonsense TBC1D4 p.Arg684Ter variant leading to loss of the muscle-specific isoform of TBC1D4 and an approximately tenfold increased risk of type 2 diabetes1. Here we show the metabolic consequences of this variant in four female and four male homozygous carriers and matched controls. An extended glucose tolerance test reveals prolonged hyperglycaemia followed by reactive hypoglycaemia in the carriers. Whole-body glucose disposal is impaired during euglycaemic-hyperinsulinaemic clamp conditions and associates with severe insulin resistance in skeletal muscle only. Notably, a marked reduction in muscle glucose transporter GLUT4 and associated proteins is observed. While metabolic regulation during exercise remains normal, the insulin-sensitizing effect of a single exercise bout is compromised. Thus, loss of the muscle-specific isoform of TBC1D4 causes severe skeletal muscle insulin resistance without baseline hyperinsulinaemia. However, physical activity can ameliorate this condition. These observations offer avenues for personalized interventions and targeted preventive strategies. In Greenlandic Inuit, a TBC1D4 loss-of-function mutation increases type 2 diabetes risk by tenfold. Carriers show severe muscle insulin resistance, impaired glucose disposal and reduced muscle GLUT4, yet exercise mitigates these defects, offering potential for personalized lifestyle interventions.

TBC1D4 p.Arg684Ter 变体携带者的骨骼肌具有严重的胰岛素抵抗,但在运动过程中表现出正常的代谢反应
在格陵兰因纽特人中,有 4% 的人是 TBC1D4 p.Arg684Ter 无义基因变异的同源携带者,这种变异会导致 TBC1D4 肌肉特异性同工酶的缺失,并使罹患 2 型糖尿病的风险增加约 10 倍1。在这里,我们在四名女性和四名男性同基因携带者以及匹配的对照组中展示了这种变异的代谢后果。延长的葡萄糖耐量试验显示,携带者会出现长时间的高血糖,随后出现反应性低血糖。在优生-高胰岛素血症钳夹条件下,全身葡萄糖处置受损,仅在骨骼肌中伴有严重的胰岛素抵抗。值得注意的是,肌肉葡萄糖转运体 GLUT4 和相关蛋白明显减少。虽然运动过程中的代谢调节仍然正常,但单次运动的胰岛素敏感效应却受到了影响。因此,TBC1D4 肌肉特异性同工酶的缺失会导致严重的骨骼肌胰岛素抵抗,而不会出现基线高胰岛素血症。然而,体育锻炼可以改善这种状况。这些观察结果为个性化干预和有针对性的预防策略提供了途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


