FSH increases lipid droplet content by regulating the expression of genes related to lipid storage in Rat Sertoli cells
IF 3.8
3区 医学
Q2 CELL BIOLOGY
引用次数: 0
Abstract
Sertoli cells (SCs) are essential for appropriate spermatogenesis. From a metabolic standpoint, they catabolize glucose and provide germ cells with lactate, which is their main energy source. SCs also oxidize fatty acids (FAs), which are stored as triacylglycerides (TAGs) within lipid droplets (LDs), to fulfill their own energy requirements. On the other hand, it has been demonstrated that FSH regulates some of SCs functions, but little is known about its effect on lipid metabolism. In the present study, we aimed to analyze FSH-mediated regulation of (1) lipid storage in LDs and (2) the expression of genes involved in FAs activation and TAG synthesis and storage in SCs. SCs obtained from 20-day-old rats were cultured for different incubation periods with FSH (100 ng/ml). It was observed that FSH increased LD content and TAG levels in SCs. There were also increments in the expression of Plin1, Fabp5, Acsl1, Acsl4, Gpat3, and Dgat1, which suggests that these proteins may mediate the increase in TAGs and LDs elicited by FSH. Regarding the signaling involved in FSH actions, it was observed that dbcAMP increased LD, and H89, a PKA inhibitor, inhibited FSH stimulus. Also, dbcAMP increased Plin2, Fabp5, Acsl1, Acsl4, and Dgat1 mRNA levels, confirming a role of the cAMP/PKA pathway in the regulation of lipid storage in SCs. Altogether, these results suggest that FSH, via the cAMP/PKA pathway, regulates lipid storage in SCs ensuring the availability of substrates to satisfy their energy requirements.
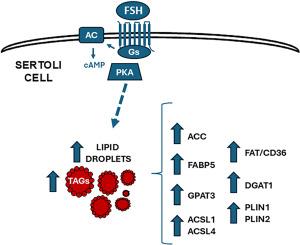
FSH 通过调节大鼠 Sertoli 细胞中脂质储存相关基因的表达增加脂滴含量
正常的精子发生离不开肥大细胞(SC)。从新陈代谢的角度来看,它们分解葡萄糖并为生殖细胞提供乳酸,这是它们的主要能量来源。生精细胞还会氧化脂肪酸(FA),以三酰甘油(TAG)的形式储存在脂滴(LD)中,以满足自身的能量需求。另一方面,已有研究表明 FSH 可调节 SCs 的某些功能,但对其对脂质代谢的影响却知之甚少。在本研究中,我们旨在分析 FSH 介导的(1)LDs 脂质储存和(2)SCs 中参与 FAs 激活和 TAG 合成及储存的基因表达的调控。用 FSH(100 毫微克/毫升)培养 20 天龄大鼠的 SCs 不同孵育期。结果发现,FSH 增加了 SCs 中的 LD 含量和 TAG 水平。Plin1、Fabp5、Acsl1、Acsl4、Gpat3和Dgat1的表达也有所增加,这表明这些蛋白可能介导了FSH引起的TAG和LD的增加。关于参与 FSH 作用的信号传导,观察到 dbcAMP 增加了 LD,而 PKA 抑制剂 H89 抑制了 FSH 的刺激。此外,dbcAMP 还增加了 Plin2、Fabp5、Acsl1、Acsl4 和 Dgat1 的 mRNA 水平,证实了 cAMP/PKA 通路在调节 SCs 脂质储存中的作用。总之,这些结果表明,FSH通过cAMP/PKA途径调节SCs的脂质储存,确保底物的可用性以满足其能量需求。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular and Cellular Endocrinology
医学-内分泌学与代谢
CiteScore
9.00
自引率
2.40%
发文量
174
审稿时长
42 days
期刊介绍:
Molecular and Cellular Endocrinology was established in 1974 to meet the demand for integrated publication on all aspects related to the genetic and biochemical effects, synthesis and secretions of extracellular signals (hormones, neurotransmitters, etc.) and to the understanding of cellular regulatory mechanisms involved in hormonal control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


