LGR6 protects against myocardial ischemia-reperfusion injury via suppressing necroptosis
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Regulated necrosis (necroptosis) and apoptosis are important biological features of ischemia-reperfusion (I/R) injury. However, the molecular mechanisms underlying myocardial necroptosis remain elusive. Leucine rich repeat containing G protein-coupled receptor 6 (LGR6) has been reported to play important roles in various cardiovascular disease. In this study, we aimed to determine whether LGR6 suppresses I/R-induced myocardial necroptosis and the underlying molecular mechanisms. We generated LGR6 knockout mice and used ligation of left anterior descending coronary artery to produce an in vivo I/R model. The effects of LGR6 and its downstream molecules were subsequently identified using RNA sequencing and CHIP assays. We observed significantly downregulated LGR6 expression in hearts post myocardial I/R and cardiomyocytes post hypoxia and reoxygenation (HR). LGR6 deficiency promoted and LGR6 overexpression inhibited necroptosis and acute myocardial injury after I/R. Mechanistically, in vivo and in vitro experiments suggest that LGR6 regulates the expression of STAT2 and ZBP1 by activating the Wnt signaling pathway, thereby inhibiting cardiomyocyte necroptosis after HR. Inhibiting STAT2 and ZBP1 effectively alleviated the aggravating effect of LGR6 deficiency on myocardial necroptosis after I/R. Furthermore, activating LGR6 with RSPO3 also effectively protected mice from acute myocardial I/R injury. Our findings reveal that RSPO3-LGR6 axis downregulates the expression of STAT2 and ZBP1 through the Wnt signaling pathway, thereby inhibiting I/R-induced myocardial injury and necroptosis. Targeting the RSPO3-LGR6 axis may be a potential therapeutic strategy to treat myocardial I/R injury.
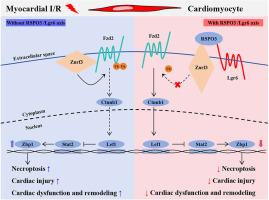
LGR6 通过抑制坏死细胞的增殖来防止心肌缺血再灌注损伤。
调节性坏死(necroptosis)和细胞凋亡是缺血再灌注(I/R)损伤的重要生物学特征。然而,心肌坏死的分子机制仍然难以捉摸。据报道,富亮氨酸重复的 G 蛋白偶联受体 6(LGR6)在各种心血管疾病中发挥着重要作用。在本研究中,我们旨在确定 LGR6 是否能抑制 I/R 诱导的心肌坏死及其潜在的分子机制。我们产生了 LGR6 基因剔除小鼠,并利用结扎左前降支冠状动脉来制作体内 I/R 模型。随后,我们利用 RNA 测序和 CHIP 分析鉴定了 LGR6 及其下游分子的影响。我们观察到,在心肌I/R后的心脏和缺氧再氧(HR)后的心肌细胞中,LGR6的表达明显下调。LGR6缺乏会促进心肌细胞坏死和急性心肌损伤,而LGR6过表达则会抑制心肌细胞坏死和急性心肌损伤。体内和体外实验从机制上表明,LGR6 通过激活 Wnt 信号通路来调节 STAT2 和 ZBP1 的表达,从而抑制 HR 后心肌细胞的坏死。抑制 STAT2 和 ZBP1 能有效缓解 LGR6 缺乏对 I/R 后心肌坏死的加重作用。此外,用 RSPO3 激活 LGR6 还能有效保护小鼠免受急性心肌 I/R 损伤。我们的研究结果表明,RSPO3-LGR6 轴可通过 Wnt 信号通路下调 STAT2 和 ZBP1 的表达,从而抑制 I/R 诱导的心肌损伤和坏死。以RSPO3-LGR6轴为靶点可能是治疗心肌I/R损伤的一种潜在治疗策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


