Designing of new functionalized imidazolium based ionic liquids attached to the antracene derivatives and investigation on the influence of intramolecular hydrogen bondings in anions on their intermolecular hydrogen bondings and some of the other properties: A DFT M06-2X-GD3 study
IF 2.7
4区 生物学
Q2 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
To promote the development of new functionalized ionic liquids, it is necessary to get a deeper insight into their features of physicochemical and electronic and molecular structure. In this study, the interaction energies and structural and vibrational frequencies parameters in accompanied with some of the physiochemical, electronic and optic attributes of ionic liquids designed by the covalently attachement of imidazolium to anthracene derivatives ([X-AnMIM][A2] and [X-AnMIM][A3], X: NH2, OH, OMe, H, Cl, CHO, CN and NO2) ILs have been evaluated. Two conjugate bases of acids 1,3,5-pentanetriol (A2) and 3-(2-hydroxyethyl)-1,3,5-pentanetriol (A3) are used as anions which have two and three intramolecular hydrogen bonds, respectively. Based on the results of calculations at M06-2X-GD3/6–311++(d,p) level of theory, the differences in these properties in addition to the structural type of anions and cations can be attributed to the cation-anion, intra and intermolecular hydrogen bonding, interactions in ionic liquids. The results depict that the ILs based on A2 anions form stronger hydrogen bonds with [X-AnMIM]+ cations. The potency of interaction between cations and anion reduces with the increasement in the number of intramolecular hydrogen bonds and also decreasement in the basic strength in the anionic part. A clear red shift is observed between [X-AnMIM][A2] and [X-AnMIM][A3] ILs and isolated anthracene, which is a clear manifestation of the effect of the imidazolium cation on the electronic energy levels of anthracene. It can be expected that the studied ILs are not electrochemically stable during the electrochemistry applications.
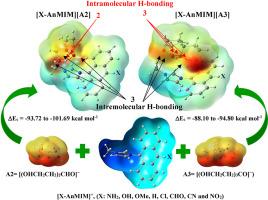
设计附着于蒽衍生物的新型官能化咪唑离子液体,并研究阴离子中分子内氢键对其分子间氢键及其他一些性质的影响:DFT M06-2X-GD3 研究。
为了促进新型功能化离子液体的开发,有必要深入了解其物理化学特征以及电子和分子结构。本研究评估了咪唑与蒽衍生物([X-AnMIM][A2] 和 [X-AnMIM][A3],X:NH2、OH、OMe、H、Cl、CHO、CN 和 NO2)共价连接设计的离子液体的相互作用能、结构和振动频率参数,以及一些物理化学、电子和光学属性。以 1,3,5-戊三醇(A2)和 3-(2-羟乙基)-1,3,5-戊三醇(A3)这两种酸的共轭碱作为阴离子,它们分别有两个和三个分子内氢键。根据 M06-2X-GD3/6-311++(d,p) 理论水平的计算结果,除了阴离子和阳离子的结构类型外,这些性质的差异还可归因于离子液体中阳离子-阴离子、分子内和分子间氢键的相互作用。结果表明,基于 A2 阴离子的离子液体能与 [X-AnMIM]+ 阳离子形成更强的氢键。阳离子和阴离子之间相互作用的效力随着分子内氢键数量的增加而降低,阴离子部分的碱性强度也随之降低。在[X-AnMIM][A2]和[X-AnMIM][A3] IL 与分离的蒽之间观察到明显的红移,这清楚地表明了咪唑阳离子对蒽电子能级的影响。可以预见,所研究的 IL 在电化学应用中并不具有电化学稳定性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of molecular graphics & modelling
生物-计算机:跨学科应用
CiteScore
5.50
自引率
6.90%
发文量
216
审稿时长
35 days
期刊介绍:
The Journal of Molecular Graphics and Modelling is devoted to the publication of papers on the uses of computers in theoretical investigations of molecular structure, function, interaction, and design. The scope of the journal includes all aspects of molecular modeling and computational chemistry, including, for instance, the study of molecular shape and properties, molecular simulations, protein and polymer engineering, drug design, materials design, structure-activity and structure-property relationships, database mining, and compound library design.
As a primary research journal, JMGM seeks to bring new knowledge to the attention of our readers. As such, submissions to the journal need to not only report results, but must draw conclusions and explore implications of the work presented. Authors are strongly encouraged to bear this in mind when preparing manuscripts. Routine applications of standard modelling approaches, providing only very limited new scientific insight, will not meet our criteria for publication. Reproducibility of reported calculations is an important issue. Wherever possible, we urge authors to enhance their papers with Supplementary Data, for example, in QSAR studies machine-readable versions of molecular datasets or in the development of new force-field parameters versions of the topology and force field parameter files. Routine applications of existing methods that do not lead to genuinely new insight will not be considered.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


