Specific ECM degradation potentiates the antitumor activity of CAR-T cells in solid tumors
IF 21.8
1区 医学
Q1 IMMUNOLOGY
引用次数: 0
Abstract
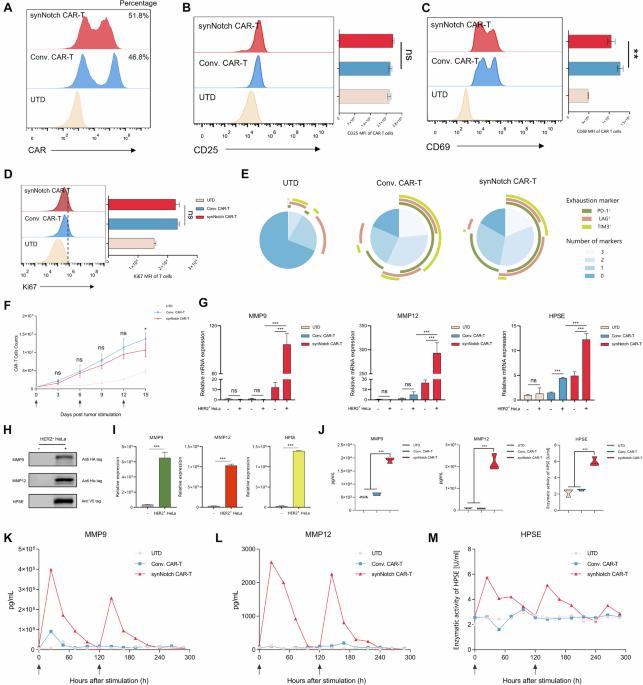
Although major progress has been made in the use of chimeric antigen receptor (CAR)-T-cell therapy for hematological malignancies, this method is ineffective against solid tumors largely because of the limited infiltration, activation and proliferation of CAR-T cells. To overcome this issue, we engineered CAR-T cells with synthetic Notch (synNotch) receptors, which induce local tumor-specific secretion of extracellular matrix (ECM)-degrading enzymes at the tumor site. SynNotch CAR-T cells achieve precise ECM recognition and robustly kill targeted tumors, with synNotch-induced enzyme production enabling the degradation of components of the tumor ECM. In addition, this regulation strongly increased the infiltration of CAR-T cells and the clearance of solid tumors, resulting in tumor regression without toxicity in vivo. Notably, synNotch CAR-T cells also promoted the persistent activation of CAR-T cells in patient-derived tumor organoids. Thus, we constructed a synthetic T-cell system that increases the infiltration and antitumor function of CAR-T cells, providing a strategy for targeting ECM-rich solid tumors.
特异性 ECM 降解可增强 CAR-T 细胞在实体瘤中的抗肿瘤活性。
尽管在使用嵌合抗原受体(CAR)-T 细胞疗法治疗血液恶性肿瘤方面取得了重大进展,但这种方法对实体瘤的治疗效果不佳,主要原因是 CAR-T 细胞的浸润、活化和增殖能力有限。为了克服这一问题,我们设计了带有合成 Notch(synNotch)受体的 CAR-T 细胞,它能在肿瘤部位诱导局部肿瘤特异性分泌细胞外基质(ECM)降解酶。SynNotch CAR-T 细胞能精确识别 ECM,并强有力地杀死靶向肿瘤,SynNotch 诱导的酶分泌能降解肿瘤 ECM 的成分。此外,这种调控还能强烈增加 CAR-T 细胞的浸润和实体瘤的清除,从而在体内实现无毒性的肿瘤消退。值得注意的是,synNotch CAR-T 细胞还能促进 CAR-T 细胞在患者衍生的肿瘤组织细胞中持续活化。因此,我们构建了一种合成 T 细胞系统,它能增强 CAR-T 细胞的浸润和抗肿瘤功能,为靶向富含 ECM 的实体瘤提供了一种策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
31.20
自引率
1.20%
发文量
903
审稿时长
1 months
期刊介绍:
Cellular & Molecular Immunology, a monthly journal from the Chinese Society of Immunology and the University of Science and Technology of China, serves as a comprehensive platform covering both basic immunology research and clinical applications. The journal publishes a variety of article types, including Articles, Review Articles, Mini Reviews, and Short Communications, focusing on diverse aspects of cellular and molecular immunology.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


