HulaCCR1, a pump-like cation channelrhodopsin discovered in a lake microbiome
IF 4.7
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Channelrhodopsins are light-gated ion channels consisting of seven transmembrane helices and a retinal chromophore, which are used as popular optogenetic tools for modulating neuronal activity. Cation channelrhodopsins (CCRs), first recognized as the photoreceptors in the chlorophyte Chlamydomonas reinhardtii, have since been identified in diverse species of green algae, as well in other unicellular eukaryotes. The CCRs from non-chlorophyte species are commonly referred to as bacteriorhodopsin-like cation channelrhodopsins, or BCCRs, as most of them feature the three characteristic amino acid residues of the “DTD motif” in the third transmembrane helix (TM3 or helix C) matching the canonical DTD motif of the well-studied archaeal light-driven proton pump bacteriorhodopsin. Here, we report characterization of HulaCCR1, a novel BCCR identified through metatranscriptomic analysis of a unicellular eukaryotic community in Lake Hula, Israel. Interestingly, HulaCCR1 has an ETD motif in which the first residue of the canonical motif is substituted for glutamate. Electrophysiological measurements of the wild-type and a mutant with a DTD motif of HulaCCR1 suggest the critical role of the first glutamate in spectral tuning and channel gating. Additionally, HulaCCR1 exhibits long extensions at the N- and C-termini. Photocurrents recorded from a truncated variant without the signal peptide predicted at the N-terminus were diminished, and membrane localization of the truncated variant significantly decreased, indicating that the signal peptide is important for membrane trafficking of HulaCCR1. These characteristics of HulaCCR1 would be related to a new biological significance in the original unidentified species, distinct from those known for other BCCRs.
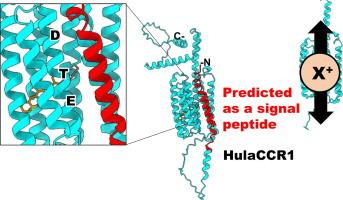
在湖泊微生物群中发现的泵状阳离子通道罗多素的扩展 N 端区和 ETD 基团的作用。
通道闪烁蛋白(Channelrhodopsins)是一种光门控离子通道,由七个跨膜螺旋和一个视网膜发色团组成,是调节神经元活动的常用光遗传工具。阳离子通道视网膜素(CCRs)最早被认为是叶绿体衣藻的光感受器,后来又在多种绿藻和其他单细胞真核生物中被发现。来自非叶绿体物种的 CCRs 通常被称为 "类细菌视紫红质通道视紫红质"(bacteriorhodopsin-like channelrhodopsins)或 BCCRs,因为它们中的大多数都具有第三个跨膜螺旋(TM3 或螺旋 C)中 "DTD 基序 "的三个特征性氨基酸残基,与已被充分研究的古生光驱动质子泵细菌视紫红质的典型 DTD 基序相匹配。在这里,我们报告了 HulaCCR1 的特征,它是通过对以色列胡拉湖中的单细胞真核生物群落进行元转录本组分析而发现的一种新型 BCCR。有趣的是,HulaCCR1 有一个 ETD 基团,其中标准基团的第一个残基被谷氨酸取代。对 HulaCCR1 的野生型和具有 DTD 基序的突变体进行的电生理测量表明,第一个谷氨酸在光谱调谐和通道门控中起着关键作用。此外,HulaCCR1 在 N 端和 C 端有较长的延伸。一个在 N 端没有信号肽的截短变体记录到的光电流减弱,截短变体的膜定位显著降低,这表明信号肽对 HulaCCR1 的膜运输非常重要。HulaCCR1 的这些特征将与原始未确定物种的新生物学意义有关,有别于已知的其他 BCCRs。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


