Regulated Proteolysis Induces Aberrant Phase Transition of Biomolecular Condensates into Aggregates: A Protective Role for the Chaperone Clusterin
IF 4.5
2区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Several proteins associated with neurodegenerative diseases, such as the mammalian prion protein (PrP), undergo liquid–liquid phase separation (LLPS), which led to the hypothesis that condensates represent precursors in the formation of neurotoxic protein aggregates. However, the mechanisms that trigger aberrant phase separation are incompletely understood. In prion diseases, protease-resistant and infectious amyloid fibrils are composed of N-terminally truncated PrP, termed C2-PrP. C2-PrP is generated by regulated proteolysis (β-cleavage) of the cellular prion protein (PrPC) specifically upon prion infection, suggesting that C2-PrP is a misfolding-prone substrate for the propagation of prions. Here we developed a novel assay to investigate the role of both LLPS and β-cleavage in the formation of C2-PrP aggregates. We show that β-cleavage induces the formation of C2-PrP aggregates, but only when full-length PrP had formed biomolecular condensates via LLPS before proteolysis. In contrast, C2-PrP remains soluble after β-cleavage of non-phase-separated PrP. To investigate whether extracellular molecular chaperones modulate LLPS of PrP and/or misfolding of C2-PrP, we focused on Clusterin. Clusterin does not inhibit LLPS of full-length PrP, however, it prevents aggregation of C2-PrP after β-cleavage of phase-separated PrP. Furthermore, Clusterin interferes with the in vitro amplification of infectious human prions isolated from Creutzfeldt-Jakob disease patients. Our study revealed that regulated proteolysis triggers aberrant phase transition of biomolecular condensates into aggregates and identified Clusterin as a component of the extracellular quality control pathway to prevent the formation and propagation of pathogenic PrP conformers.
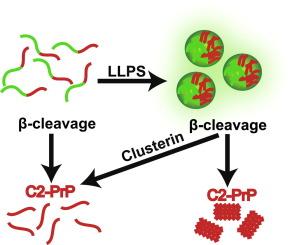
调节蛋白水解诱导生物分子凝聚物向聚集体的异常相变;伴侣蛋白Clusterin的保护作用。
与神经退行性疾病相关的几种蛋白质,如哺乳动物的朊病毒蛋白(PrP),会发生液-液相分离(LLPS),这导致了一种假设,即凝结物是神经毒性蛋白质聚集体形成的前体。然而,人们对引发异常相分离的机制尚不完全清楚。在朊病毒疾病中,抗蛋白酶和传染性淀粉样纤维由 N 端截短的 PrP(称为 C2-PrP)组成。C2-PrP是在朊病毒感染时通过细胞朊病毒蛋白(PrPC)的调节性蛋白水解(β-裂解)产生的,这表明C2-PrP是朊病毒传播过程中容易发生折叠错误的底物。在这里,我们开发了一种新的检测方法来研究 LLPS 和 β-裂解在 C2-PrP 聚集体形成过程中的作用。我们发现,β-裂解会诱导 C2-PrP 聚集体的形成,但只有当全长 PrP 在蛋白水解前已通过 LLPS 形成生物分子凝聚物时才会发生。与此相反,非相分离的 PrP 被β-裂解后,C2-PrP 仍保持可溶性。为了研究细胞外分子伴侣是否会调节 PrP 的 LLPS 和/或 C2-PrP 的错误折叠,我们重点研究了 Clusterin。Clusterin不能抑制全长PrP的LLPS,但它能防止相分离PrP的β-裂解后C2-PrP的聚集。此外,Clusterin 还能干扰从克雅氏症患者体内分离出的传染性人类朊病毒的体外扩增。我们的研究揭示了受调控的蛋白水解引发生物分子凝聚物向聚集体的异常相变,并确定了Clusterin是细胞外质量控制途径的一个组成部分,可防止致病性PrP构象的形成和传播。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Biology
生物-生化与分子生物学
CiteScore
11.30
自引率
1.80%
发文量
412
审稿时长
28 days
期刊介绍:
Journal of Molecular Biology (JMB) provides high quality, comprehensive and broad coverage in all areas of molecular biology. The journal publishes original scientific research papers that provide mechanistic and functional insights and report a significant advance to the field. The journal encourages the submission of multidisciplinary studies that use complementary experimental and computational approaches to address challenging biological questions.
Research areas include but are not limited to: Biomolecular interactions, signaling networks, systems biology; Cell cycle, cell growth, cell differentiation; Cell death, autophagy; Cell signaling and regulation; Chemical biology; Computational biology, in combination with experimental studies; DNA replication, repair, and recombination; Development, regenerative biology, mechanistic and functional studies of stem cells; Epigenetics, chromatin structure and function; Gene expression; Membrane processes, cell surface proteins and cell-cell interactions; Methodological advances, both experimental and theoretical, including databases; Microbiology, virology, and interactions with the host or environment; Microbiota mechanistic and functional studies; Nuclear organization; Post-translational modifications, proteomics; Processing and function of biologically important macromolecules and complexes; Molecular basis of disease; RNA processing, structure and functions of non-coding RNAs, transcription; Sorting, spatiotemporal organization, trafficking; Structural biology; Synthetic biology; Translation, protein folding, chaperones, protein degradation and quality control.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


