A dye-decolorizing peroxidase from Vibrio cholerae can demetallate heme
IF 3.8
2区 化学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Iron is an essential element for bacterial survival. Bacterial pathogens have therefore developed methods to obtain iron. Vibrio cholerae, the intestinal pathogen that causes cholera, utilizes heme as an iron source. DyP from V. cholerae (VcDyP) is a dye-decolorizing peroxidase. When VcDyP was expressed in Escherichia coli and purified, it was found to contain protoporphyrin IX (PPIX) but not heme, indicating that the protein possesses deferrochelatase activity. Here, we examined the demetallation reaction of VcDyP using fluorescence spectroscopy. Treatment of heme-reconstituted VcDyP with sodium dithionite under anaerobic conditions led to an increase in the fluorescence intensity at 624 nm, suggesting the formation of PPIX. Although the same reaction was conducted using myoglobin, horseradish peroxidase and hemin, no increase in the fluorescence was observed. Therefore, demetallation of heme is specific to VcDyP. This reaction was faster at lower pH, but the amplitudes of the fluorescence increase were larger at pH 6.5–7.5, in clear contrast to the dye-decolorizing activity with the optimal pH of 4.5. In contrast to HutZ from V. cholerae, which is a heme-degrading enzyme that cleaves the heme macrocycle to release iron, VcDyP can remove iron from heme without degradation. To our knowledge, VcDyP is the first enzyme whose demetallation activity has been confirmed at neutral pH. Our results show that VcDyP is a bifunctional protein that degrades anthraquinone dyes and demetallates heme.
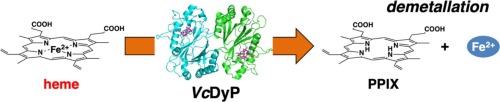
霍乱弧菌的一种染料脱色过氧化物酶可以脱金属血红素。
铁是细菌生存的基本要素。因此,细菌病原体开发出了获取铁的方法。霍乱弧菌是导致霍乱的肠道病原体,它利用血红素作为铁源。霍乱弧菌的 DyP(VcDyP)是一种染料脱色过氧化物酶。当 VcDyP 在大肠杆菌中表达并纯化时,发现它含有原卟啉 IX(PPIX),但不含血红素,这表明该蛋白具有脱赭螯合酶活性。在此,我们利用荧光光谱法研究了 VcDyP 的脱金属反应。在厌氧条件下用连二亚硫酸钠处理血红素重组的 VcDyP,在 624 纳米波长处的荧光强度增加,表明形成了 PPIX。虽然使用肌红蛋白、辣根过氧化物酶和 hemin 进行了相同的反应,但没有观察到荧光增加。因此,血红素的脱金属作用是 VcDyP 特有的。这种反应在 pH 值较低时更快,但在 pH 值为 6.5-7.5 时,荧光增加的幅度更大,这与最佳 pH 值为 4.5 时的染料脱色活性形成了明显的对比。霍乱弧菌中的 HutZ 是一种血红素降解酶,可裂解血红素大环释放铁,与之相反,VcDyP 可从血红素中去除铁而不发生降解。据我们所知,VcDyP 是第一个在中性 pH 值下被证实具有脱金属活性的酶。我们的研究结果表明,VcDyP 是一种具有降解蒽醌染料和脱金属血红素双重功能的蛋白质。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
Journal of Inorganic Biochemistry
生物-生化与分子生物学
CiteScore
7.00
自引率
10.30%
发文量
336
审稿时长
41 days
期刊介绍:
The Journal of Inorganic Biochemistry is an established international forum for research in all aspects of Biological Inorganic Chemistry. Original papers of a high scientific level are published in the form of Articles (full length papers), Short Communications, Focused Reviews and Bioinorganic Methods. Topics include: the chemistry, structure and function of metalloenzymes; the interaction of inorganic ions and molecules with proteins and nucleic acids; the synthesis and properties of coordination complexes of biological interest including both structural and functional model systems; the function of metal- containing systems in the regulation of gene expression; the role of metals in medicine; the application of spectroscopic methods to determine the structure of metallobiomolecules; the preparation and characterization of metal-based biomaterials; and related systems. The emphasis of the Journal is on the structure and mechanism of action of metallobiomolecules.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


