Miguel Fontecha-Barriuso, Natalia Villar-Gomez, Juan Guerrero-Mauvecin, Julio M Martinez-Moreno, Susana Carrasco, Diego Martin-Sanchez, María Rodríguez-Laguna, Manuel J Gómez, María D Sanchez-Niño, Marta Ruiz-Ortega, Alberto Ortiz, Ana B Sanz
下载PDF
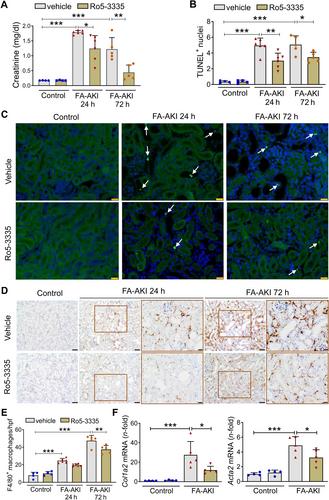
{"title":"Runt-related transcription factor 1 (RUNX1) is a mediator of acute kidney injury","authors":"Miguel Fontecha-Barriuso, Natalia Villar-Gomez, Juan Guerrero-Mauvecin, Julio M Martinez-Moreno, Susana Carrasco, Diego Martin-Sanchez, María Rodríguez-Laguna, Manuel J Gómez, María D Sanchez-Niño, Marta Ruiz-Ortega, Alberto Ortiz, Ana B Sanz","doi":"10.1002/path.6355","DOIUrl":null,"url":null,"abstract":"<p>Treatment for acute kidney injury (AKI) is suboptimal. A better understanding of the pathogenesis of AKI may lead to new therapeutic approaches. Kidney transcriptomics of folic acid-induced AKI (FA-AKI) in mice identified <i>Runx1</i> as the most upregulated RUNX family gene. We then examined the expression of RUNX1 in FA-AKI, in bacterial lipopolysaccharide (LPS)-induced cytokine storm-AKI (CS-AKI), and in human AKI. In cultured mouse tubule cells, we explored the expression and role of RUNX1 in response to the cytokine TWEAK or LPS. A chemical inhibitor of RUNX1 (Ro5-3335) was used in animal models of AKI to test its potential as a therapeutic target. RUNX1 overexpression in FA-AKI was validated at the mRNA and protein levels and localized mainly to tubule cell nuclei. CS-AKI also upregulated kidney RUNX1. Increased tubule and interstitial RUNX1 expression were also observed in human AKI. In cultured mouse tubule cells, the pro-inflammatory cytokine TWEAK and LPS increased RUNX1 and IL-6 expression. Mechanistically, RUNX1 bound to the <i>Il6</i> gene promoter and RUNX1 targeting with the chemical inhibitor Ro5-3335, or a specific small interfering RNA (siRNA), prevented the TWEAK- and LPS-induced upregulation of IL6 through a RUNX1/NFκB1 p50 pathway. <i>In vivo</i>, preventive Ro5-3335 improved kidney function and reduced inflammation in FA-AKI and CS-AKI. However, Ro5-3335 administration after the insult only improved kidney function in CS-AKI. Kidney transcriptomics identified inflammatory genes and transcription factor mRNAs such as <i>Yap1</i> and <i>Trp53</i> as key targets of Ro5-3335 in CS-AKI. In conclusion, RUNX1 contributes to AKI by driving the expression of genes involved in inflammation and represents a novel therapeutic target in AKI. © 2024 The Author(s). <i>The Journal of Pathology</i> published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.</p>","PeriodicalId":232,"journal":{"name":"The Journal of Pathology","volume":"264 4","pages":"396-410"},"PeriodicalIF":5.6000,"publicationDate":"2024-10-29","publicationTypes":"Journal Article","fieldsOfStudy":null,"isOpenAccess":false,"openAccessPdf":"https://onlinelibrary.wiley.com/doi/epdf/10.1002/path.6355","citationCount":"0","resultStr":null,"platform":"Semanticscholar","paperid":null,"PeriodicalName":"The Journal of Pathology","FirstCategoryId":"3","ListUrlMain":"https://onlinelibrary.wiley.com/doi/10.1002/path.6355","RegionNum":2,"RegionCategory":"医学","ArticlePicture":[],"TitleCN":null,"AbstractTextCN":null,"PMCID":null,"EPubDate":"","PubModel":"","JCR":"Q1","JCRName":"ONCOLOGY","Score":null,"Total":0}
引用次数: 0
引用
批量引用
Abstract
Treatment for acute kidney injury (AKI) is suboptimal. A better understanding of the pathogenesis of AKI may lead to new therapeutic approaches. Kidney transcriptomics of folic acid-induced AKI (FA-AKI) in mice identified Runx1 as the most upregulated RUNX family gene. We then examined the expression of RUNX1 in FA-AKI, in bacterial lipopolysaccharide (LPS)-induced cytokine storm-AKI (CS-AKI), and in human AKI. In cultured mouse tubule cells, we explored the expression and role of RUNX1 in response to the cytokine TWEAK or LPS. A chemical inhibitor of RUNX1 (Ro5-3335) was used in animal models of AKI to test its potential as a therapeutic target. RUNX1 overexpression in FA-AKI was validated at the mRNA and protein levels and localized mainly to tubule cell nuclei. CS-AKI also upregulated kidney RUNX1. Increased tubule and interstitial RUNX1 expression were also observed in human AKI. In cultured mouse tubule cells, the pro-inflammatory cytokine TWEAK and LPS increased RUNX1 and IL-6 expression. Mechanistically, RUNX1 bound to the Il6 gene promoter and RUNX1 targeting with the chemical inhibitor Ro5-3335, or a specific small interfering RNA (siRNA), prevented the TWEAK- and LPS-induced upregulation of IL6 through a RUNX1/NFκB1 p50 pathway. In vivo , preventive Ro5-3335 improved kidney function and reduced inflammation in FA-AKI and CS-AKI. However, Ro5-3335 administration after the insult only improved kidney function in CS-AKI. Kidney transcriptomics identified inflammatory genes and transcription factor mRNAs such as Yap1 and Trp53 as key targets of Ro5-3335 in CS-AKI. In conclusion, RUNX1 contributes to AKI by driving the expression of genes involved in inflammation and represents a novel therapeutic target in AKI. © 2024 The Author(s). The Journal of Pathology published by John Wiley & Sons Ltd on behalf of The Pathological Society of Great Britain and Ireland.
Runt 相关转录因子 1 (RUNX1) 是急性肾损伤的介质。
急性肾损伤(AKI)的治疗效果并不理想。更好地了解 AKI 的发病机制可能会带来新的治疗方法。叶酸诱导的急性肾损伤(FA-AKI)小鼠肾脏转录组学发现,RUNX1是上调最多的RUNX家族基因。我们随后研究了RUNX1在叶酸诱导的AKI、细菌脂多糖(LPS)诱导的细胞因子风暴-AKI(CS-AKI)和人类AKI中的表达。在培养的小鼠肾小管细胞中,我们探讨了 RUNX1 在细胞因子 TWEAK 或 LPS 诱导的反应中的表达和作用。在 AKI 动物模型中使用了 RUNX1 化学抑制剂(Ro5-3335),以测试其作为治疗靶点的潜力。RUNX1在FA-AKI中的过表达在mRNA和蛋白质水平上得到了验证,并且主要定位于肾小管细胞核。CS-AKI 也上调了肾脏 RUNX1。在人类 AKI 中也观察到肾小管和肾间质 RUNX1 表达增加。在培养的小鼠肾小管细胞中,促炎细胞因子 TWEAK 和 LPS 增加了 RUNX1 和 IL-6 的表达。从机制上讲,RUNX1与Il6基因启动子结合,用化学抑制剂Ro5-3335或特异性小干扰RNA(siRNA)靶向RUNX1,可通过RUNX1/NFκB1 p50途径阻止TWEAK和LPS诱导的IL6上调。在体内,预防性 Ro5-3335 可改善 FA-AKI 和 CS-AKI 的肾功能并减轻炎症反应。然而,在损伤后服用Ro5-3335只能改善CS-AKI的肾功能。肾脏转录组学发现,炎症基因和转录因子 mRNA(如 Yap1 和 Trp53)是 Ro5-3335 在 CS-AKI 中的关键靶点。总之,RUNX1通过驱动炎症相关基因的表达促进了AKI,是AKI的一个新的治疗靶点。© 2024 作者。病理学杂志》由 John Wiley & Sons Ltd 代表大不列颠及爱尔兰病理学会出版。
本文章由计算机程序翻译,如有差异,请以英文原文为准。

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


