Phosphine-Catalyzed [3 + 4] Annulations of Salicylaldehyde Schiff Bases with α-Substituted Allenes: Construction of Functionalized Benzoxepine Fused Succinimide Derivatives
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
Herein we reported a novel strategy for constructing benzoxepine fused succinimide derivatives via a phosphine-catalyzed [3 + 4] cyclization of α-substituted allenes and salicylaldehyde Schiff bases. This methodology serves as a conduit for the construction of benzoxepine derivatives in good yields under mild conditions by an unprecedented mode involving the β′-carbon of allenes. Density functional theory calculations were conducted to study the possible mechanism. Moreover, this class of compounds exhibited the potential ability of cytotoxicity toward cancer cells.
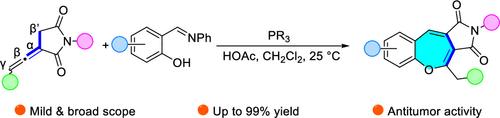
磷化氢催化水杨醛席夫碱与α-取代烯烃的[3 + 4]嵌合反应:构建功能化苯并氧杂环庚烷融合丁二酰亚胺衍生物
在此,我们报告了一种通过膦催化α-取代的烯和水杨醛席夫碱的[3 + 4]环化反应来构建苯并氧西平融合琥珀酰亚胺衍生物的新策略。这种方法是在温和条件下,通过涉及烯的β′-碳的一种前所未有的模式,以良好的产率构建苯并氧杂卓衍生物的一种途径。研究人员通过密度泛函理论计算研究了可能的机理。此外,这类化合物对癌细胞具有潜在的细胞毒性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


