Results from the autoPET challenge on fully automated lesion segmentation in oncologic PET/CT imaging
IF 18.8
1区 计算机科学
Q1 COMPUTER SCIENCE, ARTIFICIAL INTELLIGENCE
引用次数: 0
Abstract
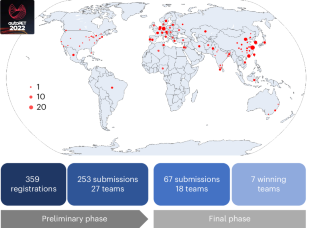
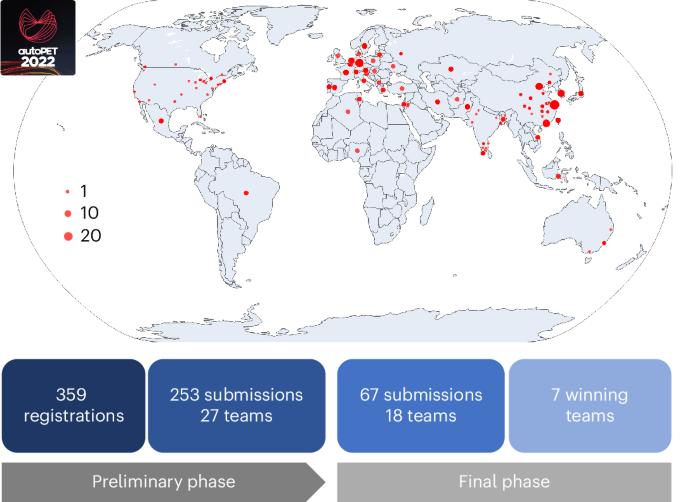
Automated detection of tumour lesions on positron emission tomography–computed tomography (PET/CT) image data is a clinically relevant but highly challenging task. Progress in this field has been hampered in the past owing to the lack of publicly available annotated data and limited availability of platforms for inter-institutional collaboration. Here we describe the results of the autoPET challenge, a biomedical image analysis challenge aimed to motivate research in the field of automated PET/CT image analysis. The challenge task was the automated segmentation of metabolically active tumour lesions on whole-body 18F-fluorodeoxyglucose PET/CT. Challenge participants had access to a large publicly available annotated PET/CT dataset for algorithm training. All algorithms submitted to the final challenge phase were based on deep learning methods, mostly using three-dimensional U-Net architectures. Submitted algorithms were evaluated on a private test set composed of 150 PET/CT studies from two institutions. An ensemble model of the highest-ranking algorithms achieved favourable performance compared with individual algorithms. Algorithm performance was dependent on the quality and quantity of data and on algorithm design choices, such as tailored post-processing of predicted segmentations. Future iterations of this challenge will focus on generalization and clinical translation. Automating the image analysis process for oncologic whole-body positron emission tomography–computed tomography data is a key area of interest. Gatidis et al. describe the autoPET 2022 challenge, an international competition focused on the segmentation of metabolically active tumour lesions, aiming to advance techniques in the field.
肿瘤 PET/CT 成像中全自动病灶分割的 autoPET 挑战赛结果
在正电子发射计算机断层扫描(PET/CT)图像数据上自动检测肿瘤病灶是一项与临床相关但极具挑战性的任务。过去,由于缺乏公开可用的注释数据以及机构间合作平台有限,这一领域的研究进展一直受阻。我们在此介绍 autoPET 挑战赛的结果,这是一项生物医学图像分析挑战赛,旨在激励 PET/CT 图像自动分析领域的研究。挑战任务是自动分割全身 18F 氟脱氧葡萄糖 PET/CT 上代谢活跃的肿瘤病灶。挑战赛的参赛者可以访问大量公开的注释 PET/CT 数据集,进行算法训练。提交到最后挑战阶段的所有算法都基于深度学习方法,大多使用三维 U-Net 架构。提交的算法在一个私人测试集上进行了评估,该测试集由来自两个机构的 150 项 PET/CT 研究组成。与单个算法相比,排名最高算法的集合模型取得了良好的性能。算法的性能取决于数据的质量和数量以及算法设计的选择,例如对预测分割进行量身定制的后处理。这项挑战赛的未来迭代将侧重于推广和临床转化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature Machine Intelligence
Multiple-
CiteScore
36.90
自引率
2.10%
发文量
127
期刊介绍:
Nature Machine Intelligence is a distinguished publication that presents original research and reviews on various topics in machine learning, robotics, and AI. Our focus extends beyond these fields, exploring their profound impact on other scientific disciplines, as well as societal and industrial aspects. We recognize limitless possibilities wherein machine intelligence can augment human capabilities and knowledge in domains like scientific exploration, healthcare, medical diagnostics, and the creation of safe and sustainable cities, transportation, and agriculture. Simultaneously, we acknowledge the emergence of ethical, social, and legal concerns due to the rapid pace of advancements.
To foster interdisciplinary discussions on these far-reaching implications, Nature Machine Intelligence serves as a platform for dialogue facilitated through Comments, News Features, News & Views articles, and Correspondence. Our goal is to encourage a comprehensive examination of these subjects.
Similar to all Nature-branded journals, Nature Machine Intelligence operates under the guidance of a team of skilled editors. We adhere to a fair and rigorous peer-review process, ensuring high standards of copy-editing and production, swift publication, and editorial independence.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


