Senescence suppresses the integrated stress response and activates a stress-remodeled secretory phenotype
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Senescence is a state of indefinite cell-cycle arrest associated with aging, cancer, and age-related diseases. Here, we find that translational deregulation, together with a corresponding maladaptive integrated stress response (ISR), is a hallmark of senescence that desensitizes senescent cells to stress. We present evidence that senescent cells maintain high levels of eIF2α phosphorylation, typical of ISR activation, but translationally repress production of the stress response activating transcription factor 4 (ATF4) by ineffective bypass of the inhibitory upstream open reading frames (uORFs). Surprisingly, ATF4 translation remains inhibited even after acute proteotoxic and amino acid starvation stressors, resulting in a highly diminished stress response. We also find that stress augments the senescence-associated secretory phenotype with sustained remodeling of inflammatory factors expression that is suppressed by non-uORF carrying ATF4 mRNA expression. Our results thus show that senescent cells possess a unique response to stress, which entails an increase in their inflammatory profile.
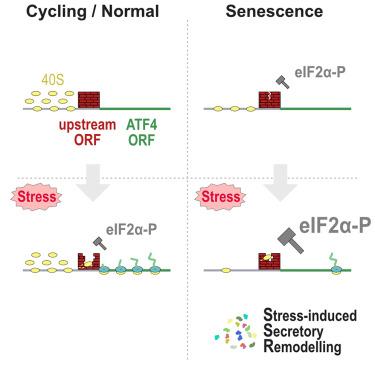
衰老抑制综合应激反应,激活应激重塑分泌表型
衰老是一种细胞周期无限期停滞的状态,与衰老、癌症和与年龄相关的疾病有关。在这里,我们发现翻译失调以及相应的适应不良综合应激反应(ISR)是衰老的一个标志,它使衰老细胞对应激脱敏。我们提出的证据表明,衰老细胞保持着高水平的eIF2α磷酸化,这是ISR激活的典型表现,但通过无效绕过抑制性上游开放阅读框(uORF),翻译抑制了应激反应激活转录因子4(ATF4)的产生。令人惊讶的是,即使在急性蛋白毒性和氨基酸饥饿胁迫下,ATF4 的翻译仍会受到抑制,从而导致应激反应的高度减弱。我们还发现,应激会增强衰老相关的分泌表型,持续重塑炎症因子的表达,而非uORF携带的ATF4 mRNA表达会抑制炎症因子的表达。因此,我们的研究结果表明,衰老细胞对应激有一种独特的反应,即增加其炎症特征。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


