Excessive MYC-topoisome activity triggers acute DNA damage, MYC degradation, and replacement by a p53-topoisome
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Hyperproliferation driven by the protooncogene MYC may lead to tumor suppressor p53 activating DNA damage that has been presumed to derive from hypertranscription and over-replication. Here, we report that excessive MYC-topoisome (MYC/topoisomerase 1/topoisomerase 2) activity acutely damages DNA-activating pATM and p53. In turn, MYC is shut off and degraded, releasing TOP1 and TOP2A from MYC topoisomes in vitro and in vivo. To manage the topological and torsional stress generated at its target genes, p53 assembles a separate topoisome. Because topoisomerase activity is intrinsically DNA damaging, p53 topoisomes provoke an initial burst of DNA damage. Because p53, unlike MYC, upregulates the DNA-damage response (DDR) and activates tyrosyl-DNA-phosphodiesterase (TDP) 1 and TDP2, it suppresses further topoisome-mediated damage. The physical coupling and activation of TOP1 and TOP2 by p53 creates a tool that supports p53-target expression while braking MYC-driven proliferation in mammalian cells.
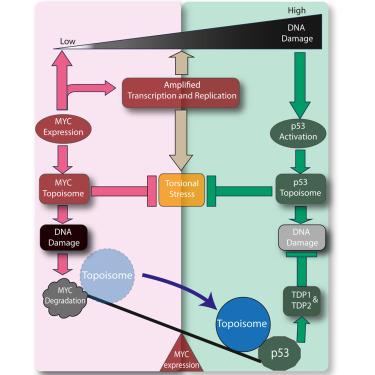
过度的 MYC-拓扑结构活性会引发急性 DNA 损伤、MYC 降解并被 p53-拓扑结构所取代
原癌基因 MYC 驱动的过度增殖可能会导致肿瘤抑制因子 p53 激活 DNA 损伤,而这种损伤被认为来自于过度转录和过度复制。在这里,我们报告了过度的 MYC-拓扑异构体(MYC/拓扑异构酶 1/拓扑异构酶 2)活性会急性损伤激活 pATM 和 p53 的 DNA。反过来,MYC 被关闭和降解,在体外和体内从 MYC 拓扑异构体中释放出 TOP1 和 TOP2A。为了管理在其靶基因上产生的拓扑和扭转压力,p53 组装了一个独立的拓扑异构体。由于拓扑异构酶活性本质上具有 DNA 损伤性,p53 拓扑体会引发最初的 DNA 损伤爆发。与 MYC 不同,p53 会上调 DNA 损伤反应(DDR)并激活酪氨酰-DNA 磷酸二酯酶(TDP)1 和 TDP2,从而抑制拓扑异构体介导的进一步损伤。p53 对 TOP1 和 TOP2 的物理耦合和激活创造了一种工具,在支持 p53 目标表达的同时,抑制哺乳动物细胞中 MYC 驱动的增殖。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


