Synthesis, characterization and in silico studies of coumarin-chalcone derivatives and their cytotoxicity activity against breast cancer cells
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Breast carcinoma disease is the most common ailment affecting women globally. Despite relentless efforts by scientists, numerous scientific strategies have been blocked by the aggressive nature of cancer cells, impeding their invasion, and spread to neighbouring tissues. This study aimed on evaluating the cytotoxic effects of newly synthesized coumarin chalcone compounds on breast cancer cell lines (MCF-7). The findings were compared with computational data derived from computer-assisted software and the Swiss ADME web tool. Molecular docking analyses indicated that all the synthesized compounds satisfied Lipinski's criteria. Furthermore, results from cytotoxicity assays demonstrated that compounds 5A, 5B, and 7A displayed significant efficacy against MCF-7 cells with IC50 (6.44 ± 0.64 μM, 8.48 ± 1.06 μM, 9.02 ± 0.96 μM), this finding indicated that the compounds highlighted above demonstrated greater cytotoxic effects against human breast cancer cells MCF-7 than the control drug Tamoxifen (IC50 = 17.42 ± 1.48 μM). Therefore, compounds 5A, 5B, and 7A could be considered promising compounds against MCF-7.
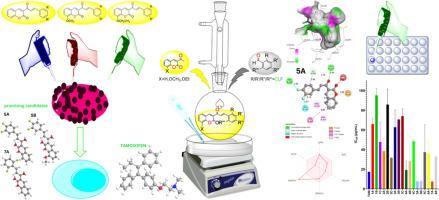
香豆素-查尔酮衍生物的合成、表征和硅学研究及其对乳腺癌细胞的细胞毒性活性
乳腺癌是全球妇女最常见的疾病。尽管科学家们做出了不懈的努力,但由于癌细胞具有侵袭性,阻碍了癌细胞向邻近组织的侵袭和扩散,许多科学策略都受到了阻碍。本研究旨在评估新合成的香豆素查尔酮化合物对乳腺癌细胞系(MCF-7)的细胞毒性作用。研究结果与来自计算机辅助软件和瑞士 ADME 网络工具的计算数据进行了比较。分子对接分析表明,所有合成的化合物都符合 Lipinski 标准。此外,细胞毒性实验结果表明,化合物 5A、5B 和 7A 对 MCF-7 细胞具有显著疗效,IC50 分别为 6.44 ± 0.64 μM、8.48 ± 1.06 μM、9.02 ± 0.96 μM。因此,化合物 5A、5B 和 7A 被认为是对 MCF-7 有希望的化合物。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


