Synthesis of dinuclear and trinuclear Ni(Ⅱ) complexes based on double-armed Salamo-type ligand containing N2O2 donors: Crystal structures, theoretical calculations, and catalytic oxidase activity
IF 4
2区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
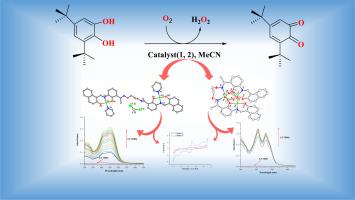
Two polynuclear Ni(II) complexes, [Ni2(L)(C5H5 N)2]⋅CH2Cl2 (1) and [Ni3(L)(μ1,3![]() OAc)2] (2), were synthesized through the reaction of a double-armed salamo-type ligand H4 L with Ni(NO3)2·6H2O (using pyridine as an auxiliary ligand) and Ni(OAc)2·4H2O, respectively. Both complexes were characterized by X-ray crystallography, PXRD, and a range of spectroscopic techniques. Crystal structure analysis revealed that complex 1 has a dinuclear linear structure with coordinated pyridine, while complex 2 exhibits a trinuclear structure with central symmetry, where two phenoxides and two acetates bridge one of the units. The acetate bridges adopt the μ2-η1: η1 coordination mode. Theoretical calculations, including molecular electrostatic potentials (MEPs) and density functional theory (DFT), were employed to predict reaction sites and assess the overall reactivity of the complexes. Both complexes 1 and 2 demonstrated catalytic activity, oxidizing 3,5-Ditert‑butyl catechol (3,5-DTBC) to the corresponding o-quinone (3,5-DTBQ), as monitored by UV-visible spectroscopy under ambient, aerobic conditions. Kinetic analysis using the Michaelis-Menten equation indicated turnover numbers (Kcat) of 599 h⁻¹ and 443 h⁻¹ for complexes 1 and 2, respectively. The mechanistic pathway of the catalytic reaction was further elucidated through ESI-mass spectrometry and cyclic voltammetry.
OAc)2] (2), were synthesized through the reaction of a double-armed salamo-type ligand H4 L with Ni(NO3)2·6H2O (using pyridine as an auxiliary ligand) and Ni(OAc)2·4H2O, respectively. Both complexes were characterized by X-ray crystallography, PXRD, and a range of spectroscopic techniques. Crystal structure analysis revealed that complex 1 has a dinuclear linear structure with coordinated pyridine, while complex 2 exhibits a trinuclear structure with central symmetry, where two phenoxides and two acetates bridge one of the units. The acetate bridges adopt the μ2-η1: η1 coordination mode. Theoretical calculations, including molecular electrostatic potentials (MEPs) and density functional theory (DFT), were employed to predict reaction sites and assess the overall reactivity of the complexes. Both complexes 1 and 2 demonstrated catalytic activity, oxidizing 3,5-Ditert‑butyl catechol (3,5-DTBC) to the corresponding o-quinone (3,5-DTBQ), as monitored by UV-visible spectroscopy under ambient, aerobic conditions. Kinetic analysis using the Michaelis-Menten equation indicated turnover numbers (Kcat) of 599 h⁻¹ and 443 h⁻¹ for complexes 1 and 2, respectively. The mechanistic pathway of the catalytic reaction was further elucidated through ESI-mass spectrometry and cyclic voltammetry.
基于含有 N2O2 给体的双臂萨拉莫型配体的双核和三核 Ni(Ⅱ)配合物的合成:晶体结构、理论计算和催化氧化酶活性
通过双臂萨拉莫型配体 H4 L 与 Ni(NO3)2-6H2O(使用吡啶作为辅助配体)和 Ni(OAc)2-4H2O 的反应,分别合成了两种多核 Ni(II) 复合物 [Ni2(L)(C5H5 N)2]⋅CH2Cl2 (1) 和 [Ni3(L)(μ1,3OAc)2] (2)。这两种配合物都通过 X 射线晶体学、PXRD 和一系列光谱技术进行了表征。晶体结构分析表明,配合物 1 具有配位吡啶的双核线性结构,而配合物 2 则具有中心对称的三核结构,其中两个苯氧基和两个醋酸盐桥接其中一个单元。醋酸桥采用 μ2-η1: η1 配位模式。理论计算包括分子静电位(MEP)和密度泛函理论(DFT),用于预测反应位点和评估配合物的整体反应性。复合物 1 和 2 都表现出催化活性,在环境有氧条件下通过紫外可见光谱将 3,5-二叔丁基邻苯二酚(3,5-DTBC)氧化成相应的邻醌(3,5-DTBQ)。利用迈克尔斯-门顿方程进行的动力学分析表明,复合物 1 和 2 的转化率(Kcat)分别为 599 h-¹ 和 443 h-¹。通过 ESI 质谱法和循环伏安法进一步阐明了催化反应的机理途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Molecular Structure
化学-物理化学
CiteScore
7.10
自引率
15.80%
发文量
2384
审稿时长
45 days
期刊介绍:
The Journal of Molecular Structure is dedicated to the publication of full-length articles and review papers, providing important new structural information on all types of chemical species including:
• Stable and unstable molecules in all types of environments (vapour, molecular beam, liquid, solution, liquid crystal, solid state, matrix-isolated, surface-absorbed etc.)
• Chemical intermediates
• Molecules in excited states
• Biological molecules
• Polymers.
The methods used may include any combination of spectroscopic and non-spectroscopic techniques, for example:
• Infrared spectroscopy (mid, far, near)
• Raman spectroscopy and non-linear Raman methods (CARS, etc.)
• Electronic absorption spectroscopy
• Optical rotatory dispersion and circular dichroism
• Fluorescence and phosphorescence techniques
• Electron spectroscopies (PES, XPS), EXAFS, etc.
• Microwave spectroscopy
• Electron diffraction
• NMR and ESR spectroscopies
• Mössbauer spectroscopy
• X-ray crystallography
• Charge Density Analyses
• Computational Studies (supplementing experimental methods)
We encourage publications combining theoretical and experimental approaches. The structural insights gained by the studies should be correlated with the properties, activity and/ or reactivity of the molecule under investigation and the relevance of this molecule and its implications should be discussed.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


