Ligand-Free Iron-Catalyzed Carbonylation of Aryl Iodides with Alkenyl Boronic Acids: Access to α,β-Unsaturated Ketones
IF 4.9
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
The application of earth-abundant and low-toxicity iron catalysts as replacements for palladium in carbonylative coupling reactions remains challenging and largely unexplored. Reported here is a highly efficient iron-catalyzed carbonylation of aryl iodides with alkenyl boronic acids under ligand-free conditions, enabling the synthesis of α,β-unsaturated ketones even at atmospheric CO pressure. The broad applicability, including its effectiveness with α-branched enones and biologically active molecules, along with high yields and selectivity, underlines the general applicability of this catalytic system.
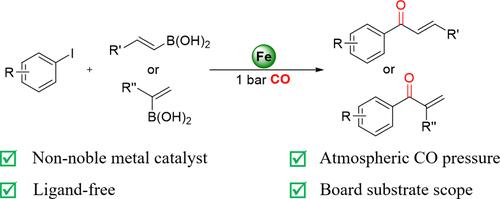
无配位铁催化芳基碘化物与烯基硼酸的羰基化反应:获得 α、β-不饱和酮体
在羰基偶联反应中应用富集于地球且毒性低的铁催化剂作为钯的替代品仍然具有挑战性,而且在很大程度上尚未得到探索。本文报告了在无配体条件下,铁催化芳基碘化物与烯基硼酸的高效羰基化反应,即使在大气 CO 压力下也能合成 α,β-不饱和酮。该催化系统具有广泛的适用性,包括对 α-支链烯酮和生物活性分子的有效性,以及高产率和高选择性,这突出表明了该催化系统的普遍适用性。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Letters
化学-有机化学
CiteScore
9.30
自引率
11.50%
发文量
1607
审稿时长
1.5 months
期刊介绍:
Organic Letters invites original reports of fundamental research in all branches of the theory and practice of organic, physical organic, organometallic,medicinal, and bioorganic chemistry. Organic Letters provides rapid disclosure of the key elements of significant studies that are of interest to a large portion of the organic community. In selecting manuscripts for publication, the Editors place emphasis on the originality, quality and wide interest of the work. Authors should provide enough background information to place the new disclosure in context and to justify the rapid publication format. Back-to-back Letters will be considered. Full details should be reserved for an Article, which should appear in due course.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


