METTL3 confers oxaliplatin resistance through the activation of G6PD-enhanced pentose phosphate pathway in hepatocellular carcinoma
IF 13.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
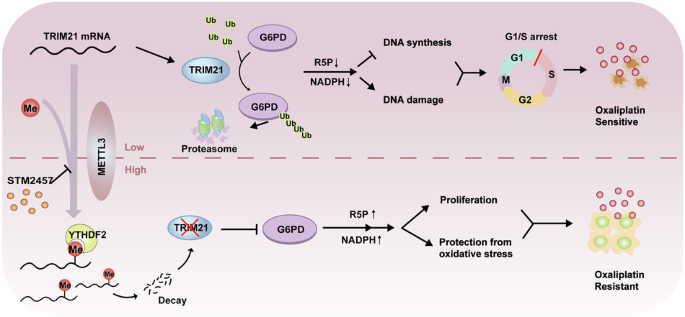
Oxaliplatin-based therapeutics is a widely used treatment approach for hepatocellular carcinoma (HCC) patients; however, drug resistance poses a significant clinical challenge. Epigenetic modifications have been implicated in the development of drug resistance. In our study, employing siRNA library screening, we identified that silencing the m6A writer METTL3 significantly enhanced the sensitivity to oxaliplatin in both in vivo and in vitro HCC models. Further investigations through combined RNA-seq and non-targeted metabolomics analysis revealed that silencing METTL3 impeded the pentose phosphate pathway (PPP), leading to a reduction in NADPH and nucleotide precursors. This disruption induced DNA damage, decreased DNA synthesis, and ultimately resulted in cell cycle arrest. Mechanistically, METTL3 was found to modify E3 ligase TRIM21 near the 3’UTR with N6-methyladenosine, leading to reduced RNA stability upon recognition by YTHDF2. TRIM21, in turn, facilitated the degradation of the rate-limiting enzyme of PPP, G6PD, through the ubiquitination-proteasome pathway. Importantly, high expression of METTL3 was significantly associated with adverse prognosis and oxaliplatin resistance in HCC patients. Notably, treatment with the specific METTL3 inhibitor, STM2457, significantly improved the efficacy of oxaliplatin. These findings underscore the critical role of the METTL3/TRIM21/G6PD axis in driving oxaliplatin resistance and present a promising strategy to overcome chemoresistance in HCC.

METTL3 通过激活肝细胞癌中 G6PD 增强的磷酸戊糖通路赋予奥沙利铂耐药性
以奥沙利铂为基础的疗法是肝细胞癌(HCC)患者广泛采用的一种治疗方法;然而,耐药性构成了重大的临床挑战。表观遗传修饰与耐药性的产生有关。在我们的研究中,通过 siRNA 文库筛选,我们发现在体内和体外 HCC 模型中,沉默 m6A 写作者 METTL3 能显著提高对奥沙利铂的敏感性。通过RNA-seq和非靶向代谢组学分析进行的进一步研究发现,沉默METTL3会阻碍磷酸戊糖途径(PPP),导致NADPH和核苷酸前体的减少。这种破坏会诱发DNA损伤,减少DNA合成,最终导致细胞周期停滞。从机理上讲,研究发现 METTL3 会用 N6-甲基腺苷修饰 3'UTR 附近的 E3 连接酶 TRIM21,导致 YTHDF2 识别后的 RNA 稳定性降低。TRIM21 反过来又促进了 PPP 的限速酶 G6PD 通过泛素化-蛋白酶体途径降解。重要的是,METTL3的高表达与HCC患者的不良预后和奥沙利铂耐药密切相关。值得注意的是,使用特异性 METTL3 抑制剂 STM2457 治疗可显著提高奥沙利铂的疗效。这些发现强调了METTL3/TRIM21/G6PD轴在驱动奥沙利铂耐药中的关键作用,并为克服HCC的化疗耐药提供了一种前景广阔的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Cell Death and Differentiation
生物-生化与分子生物学
CiteScore
24.70
自引率
1.60%
发文量
181
审稿时长
3 months
期刊介绍:
Mission, vision and values of Cell Death & Differentiation:
To devote itself to scientific excellence in the field of cell biology, molecular biology, and biochemistry of cell death and disease.
To provide a unified forum for scientists and clinical researchers
It is committed to the rapid publication of high quality original papers relating to these subjects, together with topical, usually solicited, reviews, meeting reports, editorial correspondence and occasional commentaries on controversial and scientifically informative issues.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


