AIE active Schiff base derived Pd(II) complex as a ratiometric sensor for fluoride ion
IF 2.7
3区 化学
Q2 CHEMISTRY, INORGANIC & NUCLEAR
引用次数: 0
Abstract
A tripodal AIE active Schiff base (L4) was synthesized and utilized along with pyridine for the synthesis of Pd(II) metal complex (MH1) using a two-step process. X-ray diffraction studies were performed to establish the molecular geometry of the synthesized ligand (L4) and its metal complex (MH1), which revealed square planar geometry for the Pd(II) complex. DFT studies revealed a reduction in HOMO-LUMO gap upon complexation of the ligand with Pd(II) ion. The compound L4 displayed aggregation induced emission and viscosity induced emission enhancement was observed in MH1. The developed metal complex was utilized for the sensitive and selective recognition of F− ion through a ligand exchange reaction. A ratiometric “turn-off” response along with colorimetric change are the characteristic features of the Pd(II) complex (MH1). The probe revealed a binding constant of 1.4 * 107 M−1 as calculated using Benesi-Hildebrand equation. Moreover, the binding stoichiometry in the MH1-F− complex was found to be 1:1. The sensing mechanism was established using mass spectrometry as the presence of fluoride incorporated complex was noticed. The structure of the resulting complex was also established using DFT based studies.
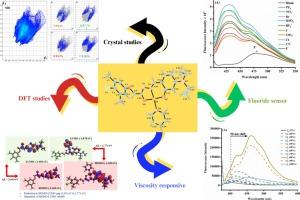
作为氟离子比率传感器的 AIE 活性席夫碱衍生钯(II)配合物
通过两步法合成了三足 AIE 活性席夫碱 (L4),并将其与吡啶一起用于合成钯(II)金属配合物 (MH1)。为了确定合成配体(L4)及其金属配合物(MH1)的分子几何形状,对其进行了 X 射线衍射研究。DFT 研究表明,配体与钯(II)离子络合后,HOMO-LUMO 间隙减小。化合物 L4 显示出聚集诱导发射,在 MH1 中观察到粘度诱导发射增强。所开发的金属复合物通过配体交换反应被用于灵敏、选择性地识别 F 离子。钯(II)配合物(MH1)的特点是具有比率 "关断 "反应和比色变化。根据贝内西-希尔德布兰德方程计算,该探针的结合常数为 1.4 * 107 M-1。此外,还发现 MH1-F- 复合物的结合比例为 1:1。由于发现了氟化物掺杂复合物的存在,因此利用质谱法确定了传感机制。此外,还利用基于 DFT 的研究确定了所得复合物的结构。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Inorganica Chimica Acta
化学-无机化学与核化学
CiteScore
6.00
自引率
3.60%
发文量
440
审稿时长
35 days
期刊介绍:
Inorganica Chimica Acta is an established international forum for all aspects of advanced Inorganic Chemistry. Original papers of high scientific level and interest are published in the form of Articles and Reviews.
Topics covered include:
• chemistry of the main group elements and the d- and f-block metals, including the synthesis, characterization and reactivity of coordination, organometallic, biomimetic, supramolecular coordination compounds, including associated computational studies;
• synthesis, physico-chemical properties, applications of molecule-based nano-scaled clusters and nanomaterials designed using the principles of coordination chemistry, as well as coordination polymers (CPs), metal-organic frameworks (MOFs), metal-organic polyhedra (MPOs);
• reaction mechanisms and physico-chemical investigations computational studies of metalloenzymes and their models;
• applications of inorganic compounds, metallodrugs and molecule-based materials.
Papers composed primarily of structural reports will typically not be considered for publication.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


