Solubility of letrozole in eight pure and five mixed solvents: Measurement, thermodynamic and molecular simulation analysis
IF 2.2
3区 工程技术
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
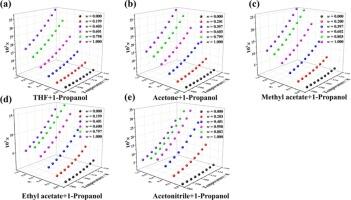
In this work, the solubility of letrozole within eight single and five binary solvents was first systematically investigated at 283.15 K to 323.15 K via the gravimetric method. Subsequently, four thermodynamics formulas including modified Apelblat, λh, GSM and Jouyban-Acree were utilized to link the experimental outcomes of letrozole, all models achieved the good fitting performance and the ARD% was less than 2 %. The dissolvability of letrozole in chosen solvents exhibited a positive correlation with temperature, and co-solvency was observed in such mixed solvent systems as ethyl acetate + 1-propanol, and acetonitrile + 1-propanol. Furthermore, the sites of hydrogen bonding donor and acceptor of letrozole and solvents were analyzed through the molecular electrostatic potential surfaces (MEPs). Then solvent effect, solvation free energy and radial distribution function (RDF) analysis gained via molecular simulation were utilized to illuminate experimental phenomena, the outcomes manifested the polarity, cohesive energy density and other properties of solvents along with both solvent–solvent and solute–solvent interactions contributed differently to the dissolution processes. In the end, the apparent thermodynamic identities concerning letrozole within chosen solvent systems were computed under the van’t Hoff and Gibbs formulas, and outcomes showed that dissolution concerning letrozole was a procedure of endothermic and entropy mainly driven.
来曲唑在八种纯溶剂和五种混合溶剂中的溶解度:测量、热力学和分子模拟分析
在这项工作中,首先通过重量法系统研究了来曲唑在 283.15 K 至 323.15 K 温度下在八种单一溶剂和五种二元溶剂中的溶解度。随后,利用改良阿佩尔布拉特公式、λh公式、GSM公式和Jouyban-Acree公式等四种热力学公式将来曲唑的实验结果联系起来,所有模型都达到了良好的拟合效果,ARD%小于2%。来曲唑在所选溶剂中的溶解度与温度呈正相关,在乙酸乙酯+1-丙醇和乙腈+1-丙醇等混合溶剂体系中观察到了共溶性。此外,还通过分子静电位面(MEPs)分析了来曲唑和溶剂的氢键供体和受体位点。结果表明,溶剂的极性、内聚能密度等特性以及溶剂与溶剂、溶剂与溶剂之间的相互作用对溶解过程产生了不同的影响。最后,根据范特霍夫公式和吉布斯公式计算了来曲唑在所选溶剂体系中的表观热力学特性,结果表明来曲唑的溶解是一个主要由内热和熵驱动的过程。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Chemical Thermodynamics
工程技术-热力学
CiteScore
5.60
自引率
15.40%
发文量
199
审稿时长
79 days
期刊介绍:
The Journal of Chemical Thermodynamics exists primarily for dissemination of significant new knowledge in experimental equilibrium thermodynamics and transport properties of chemical systems. The defining attributes of The Journal are the quality and relevance of the papers published.
The Journal publishes work relating to gases, liquids, solids, polymers, mixtures, solutions and interfaces. Studies on systems with variability, such as biological or bio-based materials, gas hydrates, among others, will also be considered provided these are well characterized and reproducible where possible. Experimental methods should be described in sufficient detail to allow critical assessment of the accuracy claimed.
Authors are encouraged to provide physical or chemical interpretations of the results. Articles can contain modelling sections providing representations of data or molecular insights into the properties or transformations studied. Theoretical papers on chemical thermodynamics using molecular theory or modelling are also considered.
The Journal welcomes review articles in the field of chemical thermodynamics but prospective authors should first consult one of the Editors concerning the suitability of the proposed review.
Contributions of a routine nature or reporting on uncharacterised materials are not accepted.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


