Electrochemical hydrogen storage using SrFe12O19 surface-immobilized polyoxometalate
IF 4.3
3区 材料科学
Q2 MATERIALS SCIENCE, MULTIDISCIPLINARY
引用次数: 0
Abstract
A tri-nickel substituted Keggin-type polyoxometalate (PMo9Ni3O37) was synthesized and subsequently immobilized on the surface of the M-type strontium hexaferrite (SrFe12O19) via the sol-gel method. The investigation of hydrogen storage capacity for the synthesized PMo9Ni3O37@SrFe12O19 nanocomposite was conducted through electrochemical methodologies employing chronopotentiometry (CP). A conventional three-electrode configuration employing copper foam coated with PMo9Ni3O37@SrFe12O19 served as the working electrode and was examined across a current range of ±1.5 mA. The charge and discharge of the experimental procedure indicated the substantial potential of the PMo9Ni3O37@SrFe12O19 nanocomposite in the hydrogen storage process, which was determined 3125 mAh/g during the discharge phase. The successful synthesis was corroborated thorough FT-IR, UV–vis, XRD, SEM, EDX, and BET surface area analysis techniques by examining the characteristic absorption wavelengths or reflection patterns. The size of the nanoparticles was determined through SEM analysis yielding a diameter of 36.76 nm, and using the Scherrer equation, a diameter of 29.22 nm was obtained.
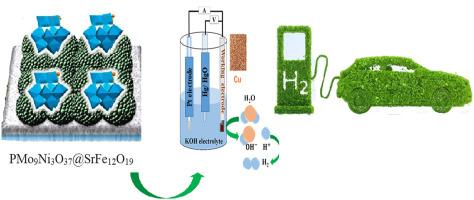
使用 SrFe12O19 表面固定的聚氧化金属酸盐进行电化学储氢
通过溶胶-凝胶法合成了三镍取代的凯金型多氧金属酸盐(PMo9Ni3O37),并随后固定在 M 型六价锶(SrFe12O19)表面。利用计时电位计(CP)的电化学方法对合成的 PMo9Ni3O37@SrFe12O19 纳米复合材料的储氢能力进行了研究。采用涂有 PMo9Ni3O37@SrFe12O19 的泡沫铜作为工作电极的传统三电极配置,在 ±1.5 mA 的电流范围内进行检测。实验过程中的充电和放电表明,PMo9Ni3O37@SrFe12O19 纳米复合材料在储氢过程中具有巨大潜力,放电阶段的储氢量为 3125 mAh/g。通过傅立叶变换红外光谱(FT-IR)、紫外可见光光谱(UV-vis)、X射线衍射光谱(XRD)、扫描电子显微镜(SEM)、电子衍射X光谱(EDX)和BET比表面积分析技术(通过检测特征吸收波长或反射模式),证实了合成的成功。通过扫描电子显微镜分析确定了纳米粒子的尺寸,得出其直径为 36.76 纳米,利用舍勒方程得出其直径为 29.22 纳米。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Materials Chemistry and Physics
工程技术-材料科学:综合
CiteScore
8.70
自引率
4.30%
发文量
1515
审稿时长
69 days
期刊介绍:
Materials Chemistry and Physics is devoted to short communications, full-length research papers and feature articles on interrelationships among structure, properties, processing and performance of materials. The Editors welcome manuscripts on thin films, surface and interface science, materials degradation and reliability, metallurgy, semiconductors and optoelectronic materials, fine ceramics, magnetics, superconductors, specialty polymers, nano-materials and composite materials.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


