Hydrogen sulfide donor NaHS inhibits formaldehyde-induced epithelial-mesenchymal transition in human lung epithelial cells via activating TGF-β1/Smad2/3 and MAPKs signaling pathways
IF 2.9
Q2 TOXICOLOGY
引用次数: 0
Abstract
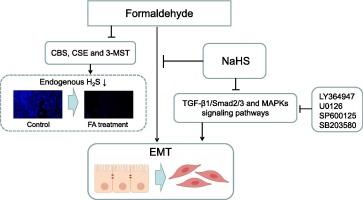
Formaldehyde (FA) long term exposure leads to abnormal pulmonary function and small airway obstruction of the patients. Hydrogen sulfide (H2S) is one of the recognized gaseous transmitters involved in a wide range of cellular functions. It is unknown the involvement of H2S in FA-induced lung injury. The purpose of this study is to investigate the therapeutic potential and mechanism of H2S on FA-induced epithelial-mesenchymal transition (EMT) of human lung epithelial cells. The cell viability of Beas2B and A549 cells after FA treatment were assessed using MTT assay. The endogenous H2S was visualized by fluorescence microscopy using of the 7-azido-4-methylcoumarin (AzMC). Cell morphology was observed under phase contrast microscope. The mRNAs and proteins level were evaluated by reverse transcription-polymerase chain reaction and western blotting assays. FA treatment downregulated the endogenous H2S levels and the mRNAs and proteins level of H2S synthesizing enzymes, such as cystathionine-β-synthase (CBS), cystathionine-γ-lyase (CSE), and 3-mercaptopyruvate sulfurtransferase (3-MST) in Beas2B and A549 cells. FA treatment changed the cell morphology of Beas2B cells from cuboid to a spindle-shape, while declined the protein level of E-cadherin and increased the protein level of Vimentin. Moreover, FA treatment increased the proteins level of transforming growth factor-β1 (TGF-β1), phosphorylated-Smad2 (p-Smad2), phosphorylated-Smad3 (p-Smad3), phosphorylated-extracellular signal-regulated kinase (p-ERK), phosphorylated-c-Jun N-terminal kinase (p-JNK), and phosphorylated-P38 (p-P38). Furthermore, the inhibitors of TGF-β receptor type 1 (TGFβRI) and mitogen-activated protein kinases (MAPKs) signaling pathways reversed FA-induced decrease in E-cadherin expression and increase in Vimentin expression in Beas2B cells. Sodium hydrogen sulfide (NaHS) increased the level of H2S, while reversed FA-induced the low expression of E-cadherin and the high expression of Vimentin, TGF-β1, p-Smad2, p-Smad3, p-ERK, p-JNK, and p-P38. These findings indicates FA treatment downregulating the endogenous H2S in human lung epithelial cells. NaHS may inhibit FA-induced EMT in human lung epithelial cells via modulating TGF-β1/Smad2/3 and MAPKs signaling pathways. Therefore, we demonstrated that supplementation of exogenous H2S may inhibit FA-induced lung injury.
硫化氢供体 NaHS 通过激活 TGF-β1/Smad2/3 和 MAPKs 信号通路抑制甲醛诱导的人肺上皮细胞上皮-间质转化
长期接触甲醛(FA)会导致患者肺功能异常和小气道阻塞。硫化氢(H2S)是公认的参与多种细胞功能的气体递质之一。目前尚不清楚 H2S 在 FA 诱导的肺损伤中的参与情况。本研究旨在探讨 H2S 对 FA 诱导的人肺上皮细胞上皮-间质转化(EMT)的治疗潜力和机制。采用MTT法评估了Beas2B和A549细胞经FA处理后的细胞活力。使用 7-叠氮-4-甲基香豆素(AzMC)通过荧光显微镜观察内源性 H2S。在相衬显微镜下观察细胞形态。通过反转录聚合酶链反应和 Western 印迹检测评估了 mRNA 和蛋白质水平。FA处理下调了Beas2B和A549细胞的内源性H2S水平以及胱硫醚-β-合成酶(CBS)、胱硫醚-γ-赖氨酸酶(CSE)和3-巯基丙酮酸硫基转移酶(3-MST)等H2S合成酶的mRNAs和蛋白质水平。FA处理使Beas2B细胞的形态从立方体变为纺锤形,同时降低了E-cadherin的蛋白水平,提高了Vimentin的蛋白水平。此外,FA 处理增加了转化生长因子-β1(TGF-β1)、磷酸化-Smad2(p-Smad2)、磷酸化-Smad3(p-Smad3)、磷酸化-细胞外信号调节激酶(p-ERK)、磷酸化-Jun N 端激酶(p-JNK)和磷酸化-P38(p-P38)的蛋白水平。此外,TGF-β受体1型(TGFβRI)和丝裂原活化蛋白激酶(MAPKs)信号通路抑制剂逆转了FA诱导的Beas2B细胞中E-cadherin表达的减少和Vimentin表达的增加。硫化氢钠(NaHS)增加了 H2S 的水平,同时逆转了 FA 诱导的 E-cadherin 低表达和 Vimentin、TGF-β1、p-Smad2、p-Smad3、p-ERK、p-JNK 和 p-P38 的高表达。这些研究结果表明,FA 会降低人肺上皮细胞中的内源性 H2S。NaHS 可通过调节 TGF-β1/Smad2/3 和 MAPKs 信号通路抑制 FA 诱导的人肺上皮细胞 EMT。因此,我们证明补充外源 H2S 可抑制 FA 诱导的肺损伤。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Current Research in Toxicology
Environmental Science-Health, Toxicology and Mutagenesis
CiteScore
4.70
自引率
3.00%
发文量
33
审稿时长
82 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


