Evaluate the potential adsorption of graphynes (perfect and doped) for nitrogen mustard gas: A first principles study
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
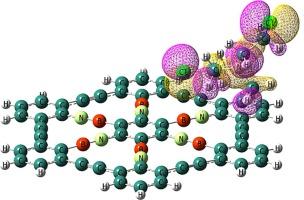
Density functional theory (DFT) calculations are used to thoroughly examine the reactivity and electronic sensitivity of pristine and BN-doped graphyne (BNG) toward nitrogen mustard (NM). Graphyne’s electrical conductivity is unaffected by the weak adsorption of NM, which occurs via the Cl atom on the material with an adsorption energy of roughly −3.1 kcal.mol−1. In addition to decreasing graphyne’s reactivity and work function, substituting isoelectronic ![]() B
B![]() N
N![]() linkages for
linkages for ![]() C
C![]() C
C![]() linkages enhances the HOMO-LUMO energy gap (Eg). BNG’s electrical conductivity increases when Eg drops from 2.99 to 1.82 eV due to the adsorption of NM. Additionally, a significant change in BNG’s work function results in a variation in the field electron emission current. Lastly, it is anticipated that the desorption of NM from the BNG surface will take a brief recovery time of roughly 0.05 s at room temperature. It has also been demonstrated that NM concentration affects changes in electrical conductivity. The findings also suggest that BNG could be a promising NM sensor.
linkages enhances the HOMO-LUMO energy gap (Eg). BNG’s electrical conductivity increases when Eg drops from 2.99 to 1.82 eV due to the adsorption of NM. Additionally, a significant change in BNG’s work function results in a variation in the field electron emission current. Lastly, it is anticipated that the desorption of NM from the BNG surface will take a brief recovery time of roughly 0.05 s at room temperature. It has also been demonstrated that NM concentration affects changes in electrical conductivity. The findings also suggest that BNG could be a promising NM sensor.
评估石墨炔(完全石墨炔和掺杂石墨炔)对氮芥气体的吸附潜力:第一原理研究
密度泛函理论(DFT)计算用于深入研究原始石墨烯和掺杂 BN 的石墨烯(BNG)对氮芥(NM)的反应性和电子敏感性。石墨的导电性不受 NM 弱吸附的影响,NM 通过材料上的 Cl 原子发生吸附,吸附能大约为 -3.1 kcal.mol-1。除了降低石墨烯的反应活性和功函数外,用等电子 BN 连接取代 CC 连接还会增强 HOMO-LUMO 能隙 (Eg)。当 Eg 从 2.99 eV 降至 1.82 eV 时,由于吸附了 NM,BNG 的导电性增加。此外,BNG 功函数的显著变化也会导致场电子发射电流的变化。最后,预计在室温下,NM 从 BNG 表面解吸将需要大约 0.05 秒的短暂恢复时间。研究还证明,NM 的浓度会影响导电率的变化。研究结果还表明,BNG 可能是一种很有前途的 NM 传感器。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


