Enhanced Corrosion-Inhibition performance of amino Gossypol: A comprehensive theoretical study
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
This study highlights the potential of amino gossypol as a green corrosion inhibitor. Comprehensive DFT calculations reveal that the electronic properties of amino gossypol, including HOMO and LUMO values, which indicate its strong electron transfer capacity and effective adsorption on steel surfaces. DFT research demonstrates a good electron transfer capacity with HOMO and LUMO values of −5.1103 eV and −0.947 eV, respectively. The study employs (molecular dynamics (MD) and Monte Carlo (MC)) simulations to investigate the interaction dynamics of amino gossypol with steel, demonstrating robust adsorption energy and the formation of a stable protective layer. The inhibitor’s adsorption energy of −65.108 Kcal/mol shows robust and spontaneous adhesion to steel, increased by its optimized molecular structure and physisorption and chemisorption methods. The substantial polarizability ( = 452.31) and specific charge distribution, with significant negative charges on oxygen atoms, facilitate efficient corrosion inhibition. Theoretical results, including reactivity indices such as chemical softness (0.4804) and electrophilicity index (2.2031), establish a strong platform for future practical investigation and possible commercial use of amino gossypol. MD simulations confirm the formation of a stable and persistent protective layer on Fe(110) surfaces. Amino gossypol is presented as an environmentally friendly and sustainable corrosion inhibitor, aligning with the growing demand for green industrial solutions. The theoretical and computational analyses predict significant corrosion inhibition performance of amino gossypol, supported by its optimized molecular structure and strong binding affinity to steel.
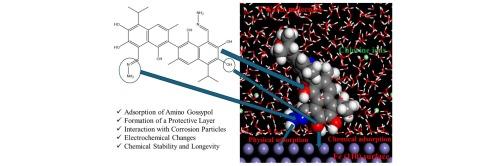
增强氨基棉酚的缓蚀性能:综合理论研究
本研究强调了氨基棉酚作为绿色缓蚀剂的潜力。全面的 DFT 计算揭示了氨基棉酚的电子特性,包括 HOMO 值和 LUMO 值,这表明它具有很强的电子转移能力并能有效地吸附在钢铁表面。DFT 研究表明,氨基棉酚具有良好的电子转移能力,其 HOMO 值为 -5.1103 eV,LUMO 值为 -0.947 eV。研究采用分子动力学(MD)和蒙特卡洛(MC)模拟来研究氨基棉酚与钢的相互作用动力学,结果表明氨基棉酚具有强大的吸附能量,并能形成稳定的保护层。抑制剂的吸附能为 -65.108 Kcal/mol,表明其对钢铁具有强大的自发吸附力,而优化的分子结构以及物理吸附和化学吸附方法提高了吸附能。其极化性(γInhDFT = 452.31)和特定电荷分布(氧原子上带有大量负电荷)有助于高效缓蚀。包括化学软度(0.4804)和亲电性指数(2.2031)等反应性指数在内的理论结果为氨基棉酚未来的实际研究和可能的商业用途建立了一个强大的平台。MD 模拟证实在 Fe(110) 表面形成了稳定持久的保护层。氨基棉酚是一种环保、可持续的缓蚀剂,符合人们对绿色工业解决方案日益增长的需求。理论和计算分析表明,氨基棉酚具有显著的缓蚀性能,这得益于其优化的分子结构和与钢的强结合亲和力。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


