The interaction between beta-ionone and 2-hydroxypropyl-beta-cyclodextrin during the formation of the inclusion complex
IF 3
3区 化学
Q3 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
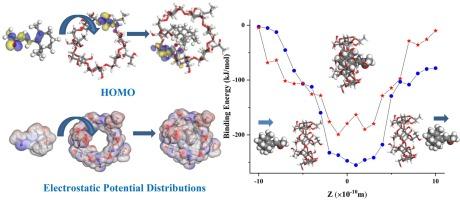
As an aroma material, beta-ionone also has a range of pharmacological activities. To solve its problems such as low bioavailability, water-immiscibility, thermo-instability, and volatility, beta-ionone was successfully encapsulated in 2-hydroxypropyl-beta-cyclodextrin (HP-beta-CD) in our previous work. However, the structure of the product, the interaction between beta-ionone and HP-beta-CD, and the mechanism of action were not clear. In this paper, in order to gain a more profound understanding of the structure and chemical behavior of beta-ionone-HP-beta-CD inclusion complex, the interaction between beta-ionone and HP-beta-CD was investigated by molecular simulation. The optimized structures, binding energy, deformation energy, charge transfer, frontier orbital energy gap, chemical hardness, chemical potential, and electrophilicity index were obtained. In the complex, beta-ionone denotes electrons to HP-beta-CD and carries a positive charge. The negative chemical potential of inclusion complex indicates that complex process is spontaneous. The complex shows a relatively higher reactivity and electrophilicity than beta-ionone and HP-beta-CD.
beta-ionone 和 2-hydroxypropyl-beta-cyclodextrin 在形成包涵复合物过程中的相互作用
作为一种芳香材料,β-酮还具有一系列药理活性。为了解决其生物利用度低、水溶性差、热不稳定性和挥发性等问题,我们在之前的研究中成功地将 beta-ionone 封装在 2-hydroxypropyl-beta-cyclodextrin (HP-beta-CD) 中。然而,该产品的结构、β-酮与 HP-beta-CD 之间的相互作用以及作用机制尚不清楚。为了更深入地了解β-酮-HP-β-CD包合物的结构和化学行为,本文通过分子模拟研究了β-酮与HP-β-CD之间的相互作用。通过分子模拟研究了β-酮与 HP-beta-CD 之间的相互作用,获得了优化的结构、结合能、变形能、电荷转移、前沿轨道能隙、化学硬度、化学势和亲电指数。在复合物中,β-酮向 HP-beta-CD 提供电子,并带有正电荷。包合络合物的化学势为负,表明络合过程是自发的。该复合物的反应活性和亲电性相对高于 beta-ionone 和 HP-beta-CD。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Computational and Theoretical Chemistry
CHEMISTRY, PHYSICAL-
CiteScore
4.20
自引率
10.70%
发文量
331
审稿时长
31 days
期刊介绍:
Computational and Theoretical Chemistry publishes high quality, original reports of significance in computational and theoretical chemistry including those that deal with problems of structure, properties, energetics, weak interactions, reaction mechanisms, catalysis, and reaction rates involving atoms, molecules, clusters, surfaces, and bulk matter.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


