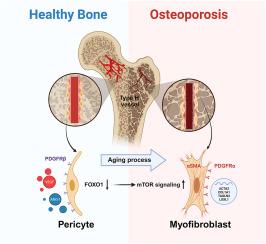
FOXO1-mTOR pathway in vascular pericyte regulates the formation of type H vessels to control bone metabolism
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
As the population aging progresses, age-related osteoporosis has become one of the most common and severe chronic degenerative diseases. Due to insufficient understanding of its complex pathomechanisms, current clinical treatments often suffer from many negative effects. Type H vessels play critical role in bone remodeling owing to their specialized function in coupling angiogenesis and osteogenesis. Increasing evidences have shown a close association between the age-related decline of type H vessels and bone loss. However, the underlying mechanisms whereby the regression of type H vessels with aging remain largely unknown.
Methods
Col2-CreERT/Foxo1flox/flox mice and FOXO1 inhibitor (AS1842856) treated adult (6 months) and middle aged (10 months) mice were utilized for evaluating the variations in bone volume, bone microarchitecture and type H vessels through micro-CT scanning analysis, histological staining and immunofluorescence staining. In vitro tube-forming and scratch assays were applied to evaluate the angiogenic capacity of human umbilical vein endothelial cells (HUVECs) exposed to AS1842856 or conditioned culture milieu of Human Brain Vascular Pericytes (HBVPs). The expression of pericyte marker proteins, myofibroblast-related proteins and genes in inhibitors-stimulated HBVPs were detected via western blot analysis and Reverse transcription-quantitative PCR (RT-qPCR). Furthermore, perivascular myofibroblastic-like transformation was confirmed in AS1842856-treated animal models through immunofluorescence staining. We also constructed Adipoq-Cre/Foxo1flox/flox conditional knockout mice and measured their bone mass and type H vessels by micro-CT and immunofluorescence staining. Mechanistic experiments in vitro were conducted via detection of mTOR signalling expression in HBVPs with pharmacological intervention (AS1842856 and rapamycin), genetic knockdown of Foxo1, or FOXO1-overexpression plasmid treatment, verified by RT-qPCR, western blot analysis and cellular immunofluorescence staining. In vivo validation was conducted on Adipoq-Cre/Foxo1flox/flox mice using immunofluorescence staining. Finally, alterations in osteo-morphology and type H vessels were verified in AS1842856-treated and rapamycin-treated aged mouse models.
Results
This study identified FOXO1 in pericytes as key components for the formation of type H vessels. We found that FOXO1 expression in pericytes decreases with aging, and pharmacological blocking with AS1842856 promoted type H vessels degeneration and increased bone loss in adult and middle-aged mice, while rapamycin prevented the above pathology in middle-aged mice. We further showed that the loss of FOXO1 in Adipoq+ pericytes led to degeneration of type H vessels and bone loss in mice. Mechanistically, the inhibition of FOXO1 by AS1842856 or knockdown of Foxo1 by siRNAs activated mTOR signaling, thereby resulting in the myofibroblastic transformation of pericytes. Furthermore, blocking mTOR signaling by rapamycin rescued the above effects in vitro and in vivo.
Conclusion
Our findings uncover a hitherto unknown role of FOXO1 in maintaining the phenotype and function of pericytes, thereby promoting formation of type H vessels. This suggests that targeting the FOXO1-mTOR pathway in pericytes could be a potential therapeutic approach to overcome the regression of type H vessels and bone degeneration with aging.
The translational potential of this article
Our research uncovers a previously unidentified role of FOXO1 in preserving pericyte characteristics and promoting the development of type H vessels. Future translational research targeting the FOXO1-mTOR pathway in pericytes may provide new strategies for the prevention and treatment of age-related osteoporosis in the clinic.
血管周细胞中的 FOXO1-mTOR 通路调节 H 型血管的形成以控制骨代谢
背景随着人口老龄化的加剧,老年性骨质疏松症已成为最常见、最严重的慢性退行性疾病之一。由于对其复杂的病理机制认识不足,目前的临床治疗往往存在许多负面影响。H 型血管在血管生成和骨生成之间具有特殊的耦合功能,因此在骨重塑过程中发挥着至关重要的作用。越来越多的证据表明,与年龄相关的 H 型血管衰退与骨质流失密切相关。方法利用Col2-CreERT/Foxo1flox/flox小鼠和FOXO1抑制剂(AS1842856)处理的成年(6个月)和中年(10个月)小鼠,通过显微CT扫描分析、组织学染色和免疫荧光染色评估骨量、骨微结构和H型血管的变化。体外成管和划痕试验用于评估暴露于 AS1842856 或人脑血管周细胞(HBVPs)条件培养液的人脐静脉内皮细胞(HUVECs)的血管生成能力。通过 Western 印迹分析和逆转录定量 PCR(RT-qPCR)检测了抑制剂刺激的 HBVPs 中的包膜标志蛋白、肌成纤维细胞相关蛋白和基因的表达。此外,在 AS1842856 处理的动物模型中,通过免疫荧光染色证实了血管周围肌成纤维细胞样转化。我们还构建了 Adipoq-Cre/Foxo1flox/flox 条件性基因敲除小鼠,并通过显微 CT 和免疫荧光染色测量了它们的骨量和 H 型血管。体外机制实验通过药物干预(AS1842856 和雷帕霉素)、基因敲除 Foxo1 或 FOXO1-overexpression 质粒处理来检测 HBVPs 中 mTOR 信号的表达,并通过 RT-qPCR、Western 印迹分析和细胞免疫荧光染色进行验证。使用免疫荧光染色对 Adipoq-Cre/Foxo1flox/flox 小鼠进行了体内验证。最后,在 AS1842856 处理和雷帕霉素处理的老龄小鼠模型中验证了骨形态和 H 型血管的改变。我们发现 FOXO1 在周细胞中的表达随着年龄的增长而减少,用 AS1842856 进行药物阻断可促进成年和中年小鼠 H 型血管的退化和骨质流失的增加,而雷帕霉素则可防止中年小鼠出现上述病理现象。我们进一步发现,Adipoq+周细胞中 FOXO1 的缺失会导致小鼠 H 型血管退化和骨质流失。从机制上讲,AS1842856抑制FOXO1或siRNA敲除Foxo1激活了mTOR信号转导,从而导致周细胞的肌成纤维细胞转化。结论:我们的发现揭示了 FOXO1 在维持周细胞的表型和功能,从而促进 H 型血管形成方面迄今未知的作用。这表明,针对周细胞中的 FOXO1-mTOR 通路可能是一种潜在的治疗方法,可以克服 H 型血管的衰退和随着衰老出现的骨退化。未来针对周细胞中FOXO1-mTOR通路的转化研究可能会为临床预防和治疗老年性骨质疏松症提供新的策略。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


