Engineered melatonin-pretreated plasma exosomes repair traumatic spinal cord injury by regulating miR-138-5p/SOX4 axis mediated microglia polarization
IF 5.9
1区 医学
Q1 ORTHOPEDICS
引用次数: 0
Abstract
Background
Neuroinflammation plays a crucial role in the repair of spinal cord injury (SCI), with microglia, pivotal in neuroinflammation, driving either degeneration or recovery in this pathological process. Recently, plasma-derived exosomes (denoted Exos) have presented a high capacity for promoting functional recovery of SCI through the anti-inflammatory effects, and pretreated exosomes are associated with better outcomes. Thus, we aimed to explore whether melatonin-pretreated plasma-derived exosomes (denoted MExo) could exert superior effects on SCI, and attempted to elucidate the potential mechanisms.
Methods
Electron microscopy, nanoparticle tracking analysis, and western blot were applied to delineate the distinctions between Exos and MExos. To assess their therapeutic potentials, we established a contusion SCI rat model, complemented by a battery of in vitro experiments comparing both groups. Subsequently, a miRNA microarray analysis was conducted, followed by a series of rescue experiments to elucidate the specific role of miRNAs in MExos. To further delve into the molecular mechanisms involved, we employed western blot analysis and the luciferase reporter gene assay.
Results
Melatonin promoted the release of exosome from plasma, concurrently amplifying their anti-inflammatory properties. Furthermore, it was discerned that MExos facilitated a transition in microglia polarization from M1 to M2 phenotype, a phenomenon more pronounced than that observed with Exos. In an endeavor to elucidate this variance, we scrutinized miRNAs exhibiting elevated expression levels in MExos, pinpointing miR-138-5p as a pivotal element in this dynamic. Following this, an in-depth investigation into the role of miR-138-5p was undertaken, which uncovered its efficacy in driving phenotypic alterations within microglia. The analysis of downstream genes targeted by miR-138-5p revealed that it exerted a negative regulatory influence on SOX4, which was found to obstruct the generation of M2-type microglia and the secretion of anti-inflammatory cytokines, thereby partially elucidating the mechanism behind miR-138-5p′s regulation of microglia polarization.
Conclusions
We innovatively observed that melatonin enhanced the anti-inflammatory function of Exos, which further decreased the expression of SOX4 by delivering miR-138-5p. This inhibition promoted the conversion of M1 microglia to M2 microglia, thus offering a viable option for the treatment of SCI.
The translational potential of this article
This study highlights that melatonin enhances the anti-inflammatory function of Exos through delivery of miR-138-5p. Activation of miR-138-5p/SOX4 axis by engineered melatonin-pretreated plasma exosomes may be a potential target for SCI treatment.
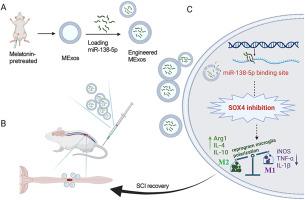
经褪黑素预处理的工程血浆外泌体通过调节 miR-138-5p/SOX4 轴介导的小胶质细胞极化修复创伤性脊髓损伤
背景神经炎症在脊髓损伤(SCI)的修复过程中起着至关重要的作用,而小胶质细胞在神经炎症中起着关键作用,它们在这一病理过程中推动着脊髓损伤的退化或恢复。最近,血浆衍生的外泌体(Exos)通过抗炎作用促进了脊髓损伤的功能恢复,预处理的外泌体与更好的预后相关。因此,我们旨在探索褪黑素预处理的血浆衍生外泌体(MExo)是否能对 SCI 发挥更好的作用,并试图阐明其潜在机制。为了评估它们的治疗潜力,我们建立了一个挫伤 SCI 大鼠模型,并通过一系列体外实验对两组模型进行了比较。随后,我们进行了 miRNA 微阵列分析,并进行了一系列挽救实验,以阐明 miRNA 在 MExos 中的特殊作用。为了进一步深入研究其中的分子机制,我们采用了 Western 印迹分析和荧光素酶报告基因实验。结果褪黑素促进了血浆中外泌体的释放,同时增强了其抗炎特性。此外,研究还发现,MExos 促进了小胶质细胞极化从 M1 型向 M2 型的转变,这一现象比 Exos 更为明显。为了阐明这种差异,我们仔细研究了在 MExos 中表达水平升高的 miRNA,发现 miR-138-5p 是这种动态变化的关键因素。随后,我们对 miR-138-5p 的作用进行了深入研究,发现了它在驱动小胶质细胞表型改变方面的功效。对 miR-138-5p 靶向的下游基因的分析表明,它对 SOX4 起着负向调控作用,而 SOX4 则阻碍了 M2 型小胶质细胞的生成和抗炎细胞因子的分泌,从而部分阐明了 miR-138-5p 对小胶质细胞极化的调控机制。结论我们创新性地观察到,褪黑激素增强了 Exos 的抗炎功能,而 Exos 通过传递 miR-138-5p 进一步降低了 SOX4 的表达。这种抑制作用促进了 M1 小胶质细胞向 M2 小胶质细胞的转化,从而为治疗 SCI 提供了一种可行的选择。经褪黑素预处理的工程血浆外泌体可激活miR-138-5p/SOX4轴,这可能是治疗SCI的一个潜在靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Journal of Orthopaedic Translation
Medicine-Orthopedics and Sports Medicine
CiteScore
11.80
自引率
13.60%
发文量
91
审稿时长
29 days
期刊介绍:
The Journal of Orthopaedic Translation (JOT) is the official peer-reviewed, open access journal of the Chinese Speaking Orthopaedic Society (CSOS) and the International Chinese Musculoskeletal Research Society (ICMRS). It is published quarterly, in January, April, July and October, by Elsevier.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


