Development and validation of a new method by MIR-FTIR and chemometrics for the early diagnosis of leprosy and evaluation of the treatment effect
IF 3.8
2区 化学
Q2 AUTOMATION & CONTROL SYSTEMS
Chemometrics and Intelligent Laboratory Systems
Pub Date : 2024-10-16
DOI:10.1016/j.chemolab.2024.105248
引用次数: 0
Abstract
Objective
Develop a new method for diagnosing leprosy and monitoring the pharmacological treatment effect of patients.
Material and methods
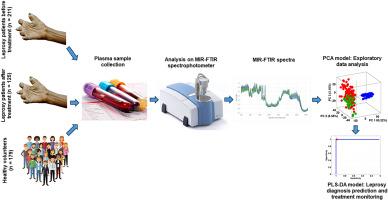
Plasma samples from patients diagnosed with leprosy (n = 211) who had not yet received any pharmacological treatment were collected at a basic health unit in Brazil. After treatment, samples were collected from the same patients (n = 125). Plasma samples from healthy volunteers were also collected (n = 179) and used as a control group. All samples were analyzed by Fourier transform mid-infrared spectrophotometry (MIR-FTIR). The spectral data of the samples were subjected to chemometric analysis. Principal component analysis (PCA) and partial least squares discriminant analysis (PLS-DA) were used to predict diagnosis and monitor pharmacological treatment.
Results
The PCA model successfully distinguished among three sample classes: healthy individuals, pre-treatment leprosy patients, and post-treatment leprosy patients. The PLS-DA algorithm accurately classified healthy, treated, and diseased samples, facilitating both reliable diagnosis and treatment monitoring for leprosy. The model achieved a sensitivity of 97 %–100 %, specificity of 100 %, and accuracy ranging from 99 % to 100 %. Furthermore, when tested on plasma samples from patients with other conditions—renal failure (n = 1032), hypertriglyceridemia (n = 100), hypercholesterolemia (n = 100), and mixed dyslipidemia (n = 100)—the model correctly classified these as negative for leprosy, with diagnostic specificity between 93 % and 96 %.
Conclusion
The MIR-FTIR technique combined with PLS-DA analysis proved to be a highly effective tool for screening leprosy patients and monitoring treatment outcomes. Given its low cost, this method could be easily implemented in laboratories across emerging and low-income countries.
利用近红外-傅立叶变换红外光谱和化学计量学开发和验证用于麻风病早期诊断和治疗效果评估的新方法
材料与方法在巴西的一家基层医疗机构采集了被诊断为麻风病但尚未接受任何药物治疗的患者(n = 211)的血浆样本。治疗后,又采集了相同患者(125 人)的血浆样本。同时还采集了健康志愿者的血浆样本(n = 179)作为对照组。所有样本均采用傅立叶变换中红外分光光度法(MIR-FTIR)进行分析。对样品的光谱数据进行了化学计量分析。主成分分析(PCA)和偏最小二乘判别分析(PLS-DA)用于预测诊断和监测药物治疗。PLS-DA 算法准确地对健康样本、治疗样本和患病样本进行了分类,为麻风病的可靠诊断和治疗监测提供了便利。该模型的灵敏度为 97%-100%,特异度为 100%,准确度为 99%-100%。此外,在对患有肾功能衰竭(n = 1032)、高甘油三酯血症(n = 100)、高胆固醇血症(n = 100)和混合型血脂异常(n = 100)的患者的血浆样本进行测试时,该模型也能正确地将这些患者归类为麻风病阴性患者,诊断特异性在 93 % 到 96 % 之间。由于成本低廉,这种方法很容易在新兴国家和低收入国家的实验室中应用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
7.50
自引率
7.70%
发文量
169
审稿时长
3.4 months
期刊介绍:
Chemometrics and Intelligent Laboratory Systems publishes original research papers, short communications, reviews, tutorials and Original Software Publications reporting on development of novel statistical, mathematical, or computer techniques in Chemistry and related disciplines.
Chemometrics is the chemical discipline that uses mathematical and statistical methods to design or select optimal procedures and experiments, and to provide maximum chemical information by analysing chemical data.
The journal deals with the following topics:
1) Development of new statistical, mathematical and chemometrical methods for Chemistry and related fields (Environmental Chemistry, Biochemistry, Toxicology, System Biology, -Omics, etc.)
2) Novel applications of chemometrics to all branches of Chemistry and related fields (typical domains of interest are: process data analysis, experimental design, data mining, signal processing, supervised modelling, decision making, robust statistics, mixture analysis, multivariate calibration etc.) Routine applications of established chemometrical techniques will not be considered.
3) Development of new software that provides novel tools or truly advances the use of chemometrical methods.
4) Well characterized data sets to test performance for the new methods and software.
The journal complies with International Committee of Medical Journal Editors'' Uniform requirements for manuscripts.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


