Predicting regional tau accumulation with machine learning-based tau-PET and advanced radiomics
Abstract
INTRODUCTION
Alzheimer's disease is partially characterized by the progressive accumulation of aggregated tau-containing neurofibrillary tangles. Although the association between accumulated tau, neurodegeneration, and cognitive decline is critical for disease understanding and clinical trial design, we still lack robust tools to predict individualized trajectories of tau accumulation. Our objective was to assess whether brain imaging biomarkers of flortaucipir-positron emission tomography (PET), in combination with clinical and genomic measures, could predict future pathological tau accumulation.
METHODS
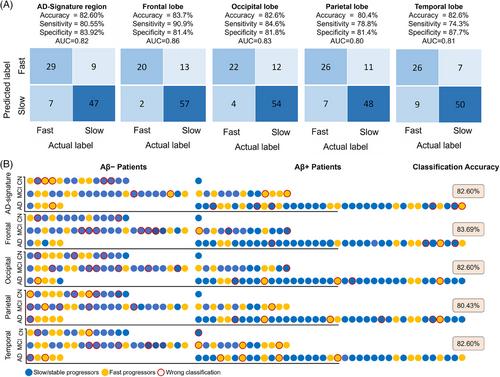
We quantified the disease profile of participants (N = 276) using a comprehensive set of descriptors, including clinical/demographic (age, diagnosis, amyloid status, sex, race, ethnicity), genetic (apolipoprotein E [APOE]-ε4), and flortaucipir-PET imaging measures (regional flortaucipir standardized uptake value ratio [SUVr] and comprehensive radiomic texture features extracted from Automated Anatomical Labeling template regions). We trained an AdaBoost machine learning algorithm in a 2:1 split train-test configuration to derive a prognostic index that (i) stratifies individualized brain regions including global (AD-signature region) and lobar regions (frontal, occipital, parietal, temporal) into stable/slow- and fast-progressors based on future tau accumulation, and (ii) forecasts individualized regional annualized-rate-of-change in flortaucipir-PET SUVr. Further, we developed an adaptive model incorporating flortaucipir-PET measurements from the baseline and intermediate timepoints to predict annualized-rate-of-change.
RESULTS
In binary classification for predicting stable/slow- versus fast-progressors, the area-under-the-receiver-operating-characteristic curve was 0.86 in the AD-signature region and 0.83, 0.82, 0.84, and 0.83 in frontal, occipital, parietal, and temporal regions, respectively. The trained models successfully predicted annualized-rate-of-change of flortaucipir-PET regional flortaucipir SUVr in AD-signature and lobar regions (Pearson-correlation [R]: AD-signature = 0.73; frontal = 0.73; occipital = 0.71; parietal = 0.70; temporal = 0.69). The models’ performance in predicting annualized-rate-of-change slightly increased when imaging features from intermediate timepoints were used in the adaptive setting (R: AD-signature = 0.79; frontal = 0.87; occipital = 0.83; parietal = 0.74; temporal = 0.82).
DISCUSSION
Taken together, our results propose a robust approach to predict future tau accumulation that may improve the ability to enroll, stratify, and gauge efficacy in clinical trial participants.
Highlights
- Machine learning predicts the future rate of tau accumulation in Alzheimer's disease.
- Tau prediction in lobar/global regions benefits from diverse multimodal features.
- This prognostic index can serve as a sensitive tool for patient stratification.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


