Thermodynamic Properties of Gadolinium Titanate Gd2Ti2O7
IF 0.7
4区 化学
Q4 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
The isobaric heat capacity of pyrochlore gadolinium titanate Gd2Ti2O7 is measured in the 2–1825 K range of temperatures. Thermodynamic functions (entropy, enthalpy change, reduced Gibbs energy) are calculated using consistent smoothed values of heat capacity. The Gibbs energy of the formation of Gd2Ti2O7 from oxides in the high temperature range is estimated.
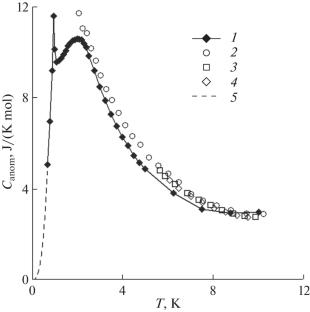
钛酸钆 Gd2Ti2O7 的热力学性质
在 2-1825 K 的温度范围内测量了热长石钆钛酸盐 Gd2Ti2O7 的等压热容。热力学函数(熵、焓变、还原吉布斯能)是利用热容量的一致平滑值计算得出的。估计了高温范围内由氧化物形成 Gd2Ti2O7 的吉布斯能。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
1.20
自引率
14.30%
发文量
376
审稿时长
5.1 months
期刊介绍:
Russian Journal of Physical Chemistry A. Focus on Chemistry (Zhurnal Fizicheskoi Khimii), founded in 1930, offers a comprehensive review of theoretical and experimental research from the Russian Academy of Sciences, leading research and academic centers from Russia and from all over the world.
Articles are devoted to chemical thermodynamics and thermochemistry, biophysical chemistry, photochemistry and magnetochemistry, materials structure, quantum chemistry, physical chemistry of nanomaterials and solutions, surface phenomena and adsorption, and methods and techniques of physicochemical studies.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


