Fundamental studies of ruthenium species supported on boron nitride nanotubes: metal loading and pretreatment effects on CO oxidation†
IF 4.2
3区 化学
Q2 CHEMISTRY, PHYSICAL
引用次数: 0
Abstract
Multiwalled boron nitride nanotube (BNNT), as a catalyst support, has become one of the promising materials due to its high oxidation resistance and thermal stability. In this work, ruthenium (Ru) supported on BNNT catalysts with different metal loadings and treatment conditions was investigated for CO oxidation as a model reaction. To understand the physicochemical properties of the prepared samples, a suite of techniques, including FTIR, UV-Raman, SEM, TEM, and XPS, was utilized. The results showed that the RuOx species were located on both the interior and the exterior surfaces of the BNNT, and an increase in metal loading led to increased active sites. 1 wt% RuOx/BNNT (oxidized) exhibited better catalytic activity than 1 wt% Ru/BNNT (reduced), indicating that treatment conditions significantly affect the catalytic properties. Reaction conditions, such as GHSV and the O2/CO ratio, were varied to further investigate the external mass transfer limitations and reaction mechanism of the 1 wt% RuOx/BNNT catalyst. The peculiar tubular morphology of the BNNT resulted in negligible external mass transfer limitation, and the catalyst might primarily follow the Eley–Rideal (ER) mechanism over the Langmuir–Hinshelwood (LH) mechanism.
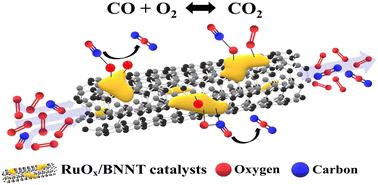
氮化硼纳米管上支持的钌物种的基础研究:金属负载和预处理对 CO 氧化的影响†。
多壁氮化硼纳米管(BNT)作为催化剂载体,因其具有高抗氧化性和热稳定性,已成为一种前景广阔的材料。本研究以 CO 氧化反应为模型,研究了不同金属负载和处理条件下 BNNT 催化剂上支撑的钌(Ru)。为了解所制备样品的理化性质,研究人员采用了一系列技术,包括傅立叶变换红外光谱、紫外-拉曼光谱、扫描电镜、电子显微镜和 XPS。结果表明,RuOx 物种位于 BNNT 的内外表面,金属负载的增加导致活性位点的增加。1 wt% RuOx/BNNT(氧化型)比 1 wt% Ru/BNNT(还原型)表现出更好的催化活性,这表明处理条件对催化特性有显著影响。为了进一步研究 1 wt% RuOx/BNNT 催化剂的外部传质限制和反应机理,改变了 GHSV 和 O2/CO 比率等反应条件。BNNT 的特殊管状形态导致外部传质限制可以忽略不计,催化剂可能主要遵循 Eley-Rideal (ER) 机制,而非 Langmuir-Hinshelwood (LH) 机制。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Catalysis Science & Technology
CHEMISTRY, PHYSICAL-
CiteScore
8.70
自引率
6.00%
发文量
587
审稿时长
1.5 months
期刊介绍:
A multidisciplinary journal focusing on cutting edge research across all fundamental science and technological aspects of catalysis.
Editor-in-chief: Bert Weckhuysen
Impact factor: 5.0
Time to first decision (peer reviewed only): 31 days
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


