Copper(ii)-catalyzed synthesis of sulfonyl-functionalized quinone-fused cyclopenta[b]indoles via four-component cascade annulation†
IF 4.7
1区 化学
Q1 CHEMISTRY, ORGANIC
引用次数: 0
Abstract
A copper-catalyzed four-component cascade annulation is realized to access novel sulfonyl-functionalized quinone-fused cyclopenta[b]indoles with high step economy and broad substrate scope, and 34 examples are constructed in one pot with up to 88% yield. This process includes sulfonyl radical triggered tandem cyclization by selective 1,1,2-trifunctionalization of terminal acetylene.
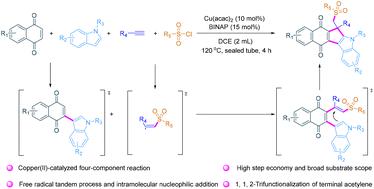
铜(II)催化的磺酰基官能化醌融合环戊并[b]吲哚四组分级联合成反应
通过铜催化的四组份级联环化反应,获得了新型磺酰基官能化醌融合环戊并[b]吲哚,具有高步经济性和广泛的底物范围。该过程包括通过选择性地对末端乙炔进行 1,1,2-三官能化而引发的磺酰基串联环化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Organic Chemistry Frontiers
CHEMISTRY, ORGANIC-
CiteScore
7.90
自引率
11.10%
发文量
686
审稿时长
1 months
期刊介绍:
Organic Chemistry Frontiers is an esteemed journal that publishes high-quality research across the field of organic chemistry. It places a significant emphasis on studies that contribute substantially to the field by introducing new or significantly improved protocols and methodologies. The journal covers a wide array of topics which include, but are not limited to, organic synthesis, the development of synthetic methodologies, catalysis, natural products, functional organic materials, supramolecular and macromolecular chemistry, as well as physical and computational organic chemistry.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


