Palmitoylation licenses RIPK1 kinase activity and cytotoxicity in the TNF pathway
IF 14.5
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Tumor necrosis factor (TNF)-induced receptor-interacting serine/threonine protein kinase 1 (RIPK1)-mediated cell death, including apoptosis and necroptosis, is increasingly recognized as a major driver of inflammatory diseases. Cell death checkpoints normally suppress RIPK1 kinase to safeguard the organism from its detrimental consequences. However, the mechanisms licensing RIPK1 kinase activity when a protective checkpoint is disabled remain unclear. Here, we identified S-palmitoylation as a licensing modification for RIPK1 kinase. TNF induces RIPK1 palmitoylation, mediated by DHHC5 and dependent on K63-linked ubiquitination of RIPK1, which enhances RIPK1 kinase activity by promoting the homo-interaction of its kinase domain and promotes cell death upon cell death checkpoint blockade. Furthermore, DHHC5 is amplified by fatty acid in the livers of mice with metabolic dysfunction-associated steatohepatitis, contributing to increased RIPK1 cytotoxicity observed in this condition. Our findings reveal that ubiquitination-dependent palmitoylation licenses RIPK1 kinase activity to induce downstream cell death signaling and suggest RIPK1 palmitoylation as a feasible target for inflammatory diseases.
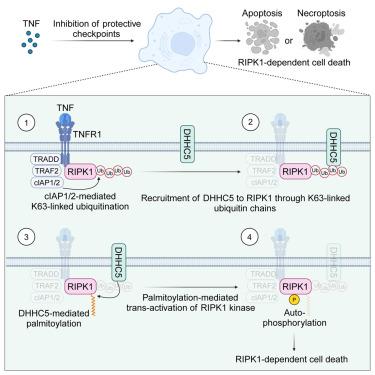
棕榈酰化许可 TNF 通路中的 RIPK1 激酶活性和细胞毒性
肿瘤坏死因子(TNF)诱导的受体丝氨酸/苏氨酸蛋白激酶 1(RIPK1)介导的细胞死亡(包括细胞凋亡和坏死)越来越被认为是炎症性疾病的主要驱动因素。细胞死亡检查点通常会抑制 RIPK1 激酶,以保护生物体免受其有害后果的影响。然而,当保护性检查点失效时,许可 RIPK1 激酶活性的机制仍不清楚。在这里,我们发现S-棕榈酰化是RIPK1激酶的一种许可修饰。TNF诱导RIPK1棕榈酰化,由DHHC5介导,依赖于RIPK1的K63连接泛素化,通过促进其激酶结构域的同源相互作用增强RIPK1激酶活性,并在细胞死亡检查点阻断后促进细胞死亡。此外,在患有代谢功能障碍相关性脂肪性肝炎的小鼠肝脏中,DHHC5会被脂肪酸放大,从而导致在这种情况下观察到的RIPK1细胞毒性增强。我们的研究结果表明,泛素化依赖的棕榈酰化作用会许可 RIPK1 激酶的活性,从而诱导下游细胞死亡信号转导,这表明 RIPK1 棕榈酰化作用是治疗炎症性疾病的一个可行靶点。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Cell
生物-生化与分子生物学
CiteScore
26.00
自引率
3.80%
发文量
389
审稿时长
1 months
期刊介绍:
Molecular Cell is a companion to Cell, the leading journal of biology and the highest-impact journal in the world. Launched in December 1997 and published monthly. Molecular Cell is dedicated to publishing cutting-edge research in molecular biology, focusing on fundamental cellular processes. The journal encompasses a wide range of topics, including DNA replication, recombination, and repair; Chromatin biology and genome organization; Transcription; RNA processing and decay; Non-coding RNA function; Translation; Protein folding, modification, and quality control; Signal transduction pathways; Cell cycle and checkpoints; Cell death; Autophagy; Metabolism.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


