Impaired striatal glutathione–ascorbate metabolism induces transient dopamine increase and motor dysfunction
IF 18.9
1区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
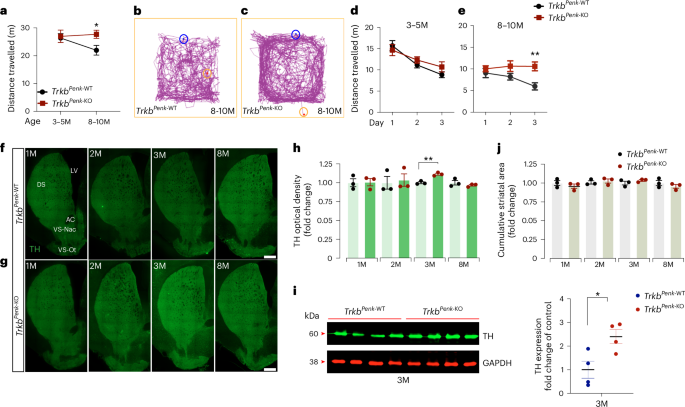
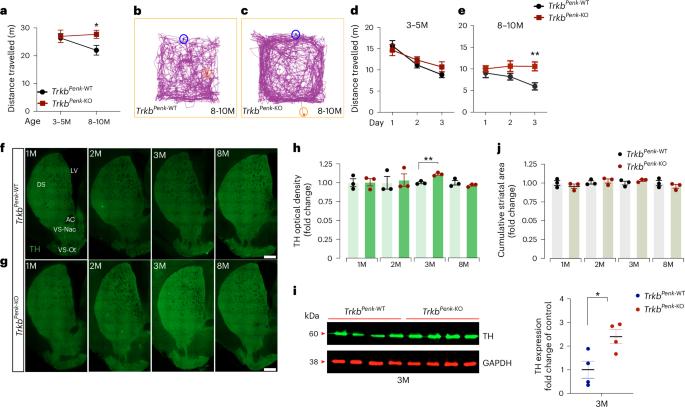
Identifying initial triggering events in neurodegenerative disorders is critical to developing preventive therapies. In Huntington’s disease (HD), hyperdopaminergia—probably triggered by the dysfunction of the most affected neurons, indirect pathway spiny projection neurons (iSPNs)—is believed to induce hyperkinesia, an early stage HD symptom. However, how this change arises and contributes to HD pathogenesis is unclear. Here, we demonstrate that genetic disruption of iSPNs function by Ntrk2/Trkb deletion in mice results in increased striatal dopamine and midbrain dopaminergic neurons, preceding hyperkinetic dysfunction. Transcriptomic analysis of iSPNs at the pre-symptomatic stage showed de-regulation of metabolic pathways, including upregulation of Gsto2, encoding glutathione S-transferase omega-2 (GSTO2). Selectively reducing Gsto2 in iSPNs in vivo effectively prevented dopaminergic dysfunction and halted the onset and progression of hyperkinetic symptoms. This study uncovers a functional link between altered iSPN BDNF-TrkB signalling, glutathione–ascorbate metabolism and hyperdopaminergic state, underscoring the vital role of GSTO2 in maintaining dopamine balance. Malik, Guo et al. show that Ntrk2/Trkb-mediated neurotrophic signalling regulates dopamine levels by controlling glutathione–ascorbate metabolism, thus impacting striatal dopaminergic circuits and motor function
纹状体谷胱甘肽-抗坏血酸代谢受损会诱发一过性多巴胺增加和运动功能障碍
确定神经退行性疾病的最初触发事件对于开发预防性疗法至关重要。在亨廷顿氏病(HD)中,多巴胺功能亢进可能是由受影响最严重的神经元--间接通路棘突投射神经元(iSPNs)--的功能障碍引发的,这被认为是诱发HD早期症状--运动过度的原因。然而,这种变化是如何产生并导致 HD 发病的还不清楚。在这里,我们证明了通过Ntrk2/Trkb缺失对小鼠iSPNs功能的遗传性破坏会导致纹状体多巴胺和中脑多巴胺能神经元的增加,进而导致过度运动功能障碍。对症状前期 iSPNs 的转录组分析表明,代谢途径失调,包括编码谷胱甘肽 S 转移酶ω-2(GSTO2)的 Gsto2 上调。在体内选择性减少 iSPNs 中的 Gsto2 可有效防止多巴胺能功能障碍,并阻止过度运动症状的出现和发展。这项研究揭示了 iSPN BDNF-TrkB 信号改变、谷胱甘肽-抗坏血酸代谢和多巴胺能亢进状态之间的功能性联系,强调了 GSTO2 在维持多巴胺平衡方面的重要作用。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Nature metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
27.50
自引率
2.40%
发文量
170
期刊介绍:
Nature Metabolism is a peer-reviewed scientific journal that covers a broad range of topics in metabolism research. It aims to advance the understanding of metabolic and homeostatic processes at a cellular and physiological level. The journal publishes research from various fields, including fundamental cell biology, basic biomedical and translational research, and integrative physiology. It focuses on how cellular metabolism affects cellular function, the physiology and homeostasis of organs and tissues, and the regulation of organismal energy homeostasis. It also investigates the molecular pathophysiology of metabolic diseases such as diabetes and obesity, as well as their treatment. Nature Metabolism follows the standards of other Nature-branded journals, with a dedicated team of professional editors, rigorous peer-review process, high standards of copy-editing and production, swift publication, and editorial independence. The journal has a high impact factor, has a certain influence in the international area, and is deeply concerned and cited by the majority of scholars.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


