Increased NPM1 inhibit ferroptosis and aggravate renal fibrosis via Nrf2 pathway in chronic kidney disease
IF 4.2
2区 生物学
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
Biochimica et biophysica acta. Molecular basis of disease
Pub Date : 2024-10-20
DOI:10.1016/j.bbadis.2024.167551
引用次数: 0
Abstract
Recent findings underscore the significance of ferroptosis, an innovative iron-dependent mode of cell death, in the etiology and progression of chronic kidney disease (CKD). Nucleophosmin 1 (NPM1), a nucleolar protein, contributes to fibrogenesis and modulates cellular functions and mortality. Initial investigations utilized bioinformatics techniques to pinpoint genes with altered expression in CKD and to forecast the potential links between NPM1, ferroptosis, and renal fibrosis. Increased NPM1 expression was verified in the renal tissues of CKD patients. Experimental models of renal fibrosis in both animals and cells were then used for further study. The suppression of NPM1 led to an augmentation in iron metabolism and lipid peroxidation processes integral to ferroptosis, contributing to the mitigation of renal fibrosis. In contrast, an elevation in NPM1 expression had the opposite effect. This modulation may be interconnected with the nuclear factor erythroid 2–related factor 2 pathway. Moreover, the application of the ferroptosis inhibitor, Fer-1, not only obstructed ferroptosis but also diminished NPM1 expression, which, in turn, contributed to the alleviation of renal fibrosis. Thus, our findings suggest that in CKD the NPM1 level increased and led to decreased ferroptosis and aggravated renal fibrosis via an Nrf2 pathway. Ferroptosis inhibitor can alleviate renal fibrosis.
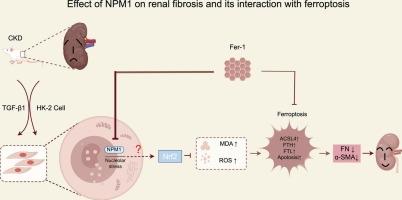
慢性肾脏病患者体内 NPM1 的增加会通过 Nrf2 途径抑制铁蛋白沉积并加重肾脏纤维化。
最近的研究结果表明,慢性肾脏病(CKD)的病因和进展中存在一种创新的铁依赖性细胞死亡模式--铁变态反应(ferroptosis)。Nucleophosmin 1(NPM1)是一种核仁蛋白,它有助于纤维形成并调节细胞功能和死亡率。最初的研究利用生物信息学技术确定了 CKD 中表达改变的基因,并预测了 NPM1、铁变态反应和肾脏纤维化之间的潜在联系。NPM1 在慢性肾功能衰竭患者肾组织中的表达增加已得到证实。然后利用动物和细胞肾纤维化实验模型进行了进一步研究。抑制 NPM1 会导致铁代谢和脂质过氧化过程的增强,从而减轻肾脏纤维化。相反,NPM1 表达的增加则产生了相反的效果。这种调节可能与核因子红细胞 2 相关因子 2 途径相互关联。此外,应用铁突变抑制剂 Fer-1 不仅能阻碍铁突变,还能减少 NPM1 的表达,这反过来又有助于减轻肾脏纤维化。因此,我们的研究结果表明,在慢性肾脏病患者中,NPM1水平升高,并通过Nrf2途径导致铁蛋白沉积减少和肾纤维化加重。铁蛋白沉积抑制剂可缓解肾脏纤维化。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
12.30
自引率
0.00%
发文量
218
审稿时长
32 days
期刊介绍:
BBA Molecular Basis of Disease addresses the biochemistry and molecular genetics of disease processes and models of human disease. This journal covers aspects of aging, cancer, metabolic-, neurological-, and immunological-based disease. Manuscripts focused on using animal models to elucidate biochemical and mechanistic insight in each of these conditions, are particularly encouraged. Manuscripts should emphasize the underlying mechanisms of disease pathways and provide novel contributions to the understanding and/or treatment of these disorders. Highly descriptive and method development submissions may be declined without full review. The submission of uninvited reviews to BBA - Molecular Basis of Disease is strongly discouraged, and any such uninvited review should be accompanied by a coverletter outlining the compelling reasons why the review should be considered.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


