A theoretical model for focal adhesion and cytoskeleton formation in non-motile cells
IF 1.9
4区 数学
Q2 BIOLOGY
引用次数: 0
Abstract
To function and survive cells need to be able to sense and respond to their local environment through mechanotransduction. Crucially, mechanical and biochemical perturbations initiate cell signalling cascades, which can induce responses such as growth, apoptosis, proliferation and differentiation. At the heart of this process are actomyosin stress fibres (SFs), which form part of the cell cytoskeleton, and focal adhesions (FAs), which bind this cytoskeleton to the extra-cellular matrix (ECM). The formation and maturation of these structures (connected by a positive feedback loop) is pivotal in non-motile cells, where SFs are generally of ventral type, interconnecting FAs and producing isometric tension. In this study we formulate a one-dimensional bio-chemo-mechanical continuum model to describe the coupled formation and maturation of ventral SFs and FAs. We use a set of reaction–diffusion–advection equations to describe three sets of biochemical events: the polymerisation of actin and subsequent bundling into activated SFs; the formation and maturation of cell–substrate adhesions; and the activation of signalling proteins in response to FA and SF formation. The evolution of these key proteins is coupled to a Kelvin–Voigt viscoelastic description of the cell cytoplasm and the ECM. We employ this model to understand how cells respond to external and intracellular cues in vitro and are able to reproduce experimentally observed phenomena including non-uniform cell striation and cells forming weaker SFs and FAs on softer substrates.
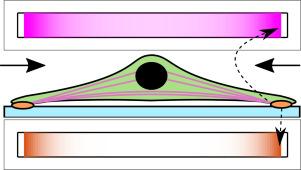
非运动细胞中病灶粘附和细胞骨架形成的理论模型。
细胞要发挥功能并存活下来,就必须能够通过机械传导来感知和响应局部环境。至关重要的是,机械和生化扰动会启动细胞信号级联,从而诱发生长、凋亡、增殖和分化等反应。这一过程的核心是构成细胞细胞骨架的肌动蛋白应力纤维(SF)和将细胞骨架与细胞外基质(ECM)结合在一起的病灶粘附(FA)。在非运动细胞中,这些结构(通过正反馈回路连接)的形成和成熟至关重要,其中 SF 通常为腹侧型,与 FA 相互连接并产生等距张力。在这项研究中,我们建立了一个一维生物化学-机械连续模型来描述腹侧 SFs 和 FAs 的耦合形成和成熟。我们使用一组反应-扩散-平流方程来描述三组生化事件:肌动蛋白的聚合和随后捆绑成活化的 SFs;细胞-基质粘附的形成和成熟;以及信号蛋白对 FA 和 SF 形成的激活反应。这些关键蛋白的进化与细胞胞质和 ECM 的开尔文-沃依格粘弹性描述相关联。我们利用这一模型来了解细胞如何在体外对外界和细胞内的线索做出反应,并能重现实验观察到的现象,包括细胞条纹不均匀以及细胞在较软的基质上形成较弱的 SF 和 FA。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.20
自引率
5.00%
发文量
218
审稿时长
51 days
期刊介绍:
The Journal of Theoretical Biology is the leading forum for theoretical perspectives that give insight into biological processes. It covers a very wide range of topics and is of interest to biologists in many areas of research, including:
• Brain and Neuroscience
• Cancer Growth and Treatment
• Cell Biology
• Developmental Biology
• Ecology
• Evolution
• Immunology,
• Infectious and non-infectious Diseases,
• Mathematical, Computational, Biophysical and Statistical Modeling
• Microbiology, Molecular Biology, and Biochemistry
• Networks and Complex Systems
• Physiology
• Pharmacodynamics
• Animal Behavior and Game Theory
Acceptable papers are those that bear significant importance on the biology per se being presented, and not on the mathematical analysis. Papers that include some data or experimental material bearing on theory will be considered, including those that contain comparative study, statistical data analysis, mathematical proof, computer simulations, experiments, field observations, or even philosophical arguments, which are all methods to support or reject theoretical ideas. However, there should be a concerted effort to make papers intelligible to biologists in the chosen field.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


