Sequence-function space of radical SAM cyclophane synthases reveal conserved active site residues that influence substrate specificity†
IF 4.2
Q2 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
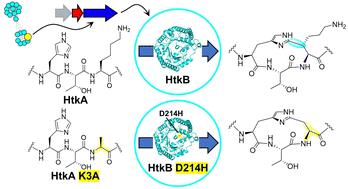
Radical SAM cyclophane synthases catalyze C–C, C–N, and C–O crosslinking reactions in the biosynthesis of bioactive peptide natural products. Here, we studied an uncharacterized rSAM enzyme, HtkB from Pandoraea sp., and found this enzyme to catalyze the formation of a HisC2-to-LysCβ crosslink. We used a combination of ColabFold and mutagenesis studies to show that residues D214 in HtkB and H204 in HaaB (another cyclophane synthase) are important for substrate specificity. Mutation of these residues changes the specificity and lowers substrate recognition on the wild-type motifs. This result opens opportunities to alter the specificity and promiscuity for rSAM peptide modifying enzymes.
自由基 SAM 环烷合成酶的序列-功能空间揭示了影响底物特异性的保守活性位点残基。
自由基 SAM 环烷合成酶可催化生物活性肽天然产物生物合成过程中的 C-C、C-N 和 C-O 交联反应。在这里,我们研究了一种未定性的 rSAM 酶,即来自 Pandoraea sp. 的 HtkB,发现这种酶能催化 HisC2 到 LysCβ 交联的形成。我们利用 ColabFold 和诱变研究相结合的方法证明,HtkB 的残基 D214 和 HaaB(另一种环烷合成酶)的残基 H204 对底物特异性非常重要。这些残基的突变改变了野生型图案的特异性并降低了底物识别率。这一结果为改变 rSAM 肽修饰酶的特异性和杂交性提供了机会。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


