Diallyl disulfide alleviates hepatic steatosis by the conservative mechanism from fish to tetrapod: Augment Mfn2/Atgl-Mediated lipid droplet-mitochondria coupling
IF 10.7
1区 生物学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Despite increasing evidences has highlighted the importance of mitochondria-lipid droplet (LD) coupling in maintaining lipid homeostasis, little progress in unraveling the role of mitochondria-LD coupling in hepatic lipid metabolism has been made. Additionally, diallyl disulfide (DADS), a garlic organosulfur compound, has been proposed to prevent hepatic steatosis; however, no studies have focused on the molecular mechanism to date. To address these gaps, this study investigated the systemic control mechanisms of mitochondria-LD coupling regulating hepatic lipid metabolism, and also explored their function in the process of DADS alleviating hepatic steatosis. To this end, an animal model of lipid metabolism, yellow catfish Pelteobagrus fulvidraco were fed four different diets (control, high-fat, DADS and high-fat + DADS diet) in vivo for 8 weeks; in vitro experiments were conducted to inhibit Mfn2/Atgl-mediated mitochondria-LD coupling in isolated hepatocytes. The key findings are: (1) the activations of hepatic LDs lipolysis and mitochondrial β-oxidation are likely the major drivers for DADS alleviating hepatic steatosis; (2) the underlying mechanism is that DADS enhances mitochondria-LD coupling by promoting the interaction between mitochondrion-localized Mfn2 with LD-localized Atgl, which facilitates the hepatic LDs lipolysis and the transfer of fatty acids (FAs) from LDs to mitochondria for subsequent β-oxidation; (3) Mfn2-mediated mitochondrial fusion facilitates mitochondria to form more PDM, which possess higher β-oxidation capacity in hepatocytes. Significantly, the present research unveils a previously undisclosed mechanism by which Mfn2/Atgl-mitochondria-LD coupling relieves hepatic LDs accumulation, which is a conserved strategy from fish to tetrapod. This study provides another dimension for mitochondria-LD coupling and opens up new avenues for the therapeutic interventions in hepatic steatosis.
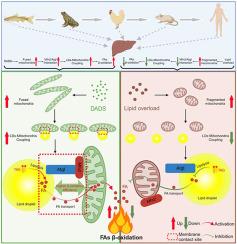
从鱼类到四足动物,二烯丙基二硫化物通过保守机制缓解肝脏脂肪变性:增强 Mfn2/Atgl 介导的脂滴-线粒体耦合。
尽管越来越多的证据强调了线粒体-脂滴(LD)耦合在维持脂质平衡中的重要性,但在揭示线粒体-LD耦合在肝脏脂质代谢中的作用方面进展甚微。此外,有人提出大蒜有机硫化合物二烯丙基二硫化物(DADS)可预防肝脏脂肪变性,但迄今为止还没有研究关注其分子机制。针对这些空白,本研究调查了线粒体-二硫化物耦联调节肝脏脂质代谢的系统控制机制,并探讨了它们在 DADS 缓解肝脂肪变性过程中的功能。为此,在体内喂食四种不同的饮食(对照组、高脂组、DADS组和高脂+DADS组)8周,以黄颡鱼为脂代谢动物模型;在体外实验中抑制离体肝细胞中Mfn2/Atgl介导的线粒体-LD偶联。主要发现有(1)肝脏 LDs 脂肪分解和线粒体 β 氧化的激活可能是 DADS 缓解肝脏脂肪变性的主要驱动力;(2)其基本机制是,DADS通过促进线粒体定位的Mfn2与LD定位的Atgl之间的相互作用,增强线粒体-LD耦联,从而促进肝脏LDs脂肪分解和脂肪酸(FA)从LDs转移到线粒体进行后续的β氧化;(3)Mfn2介导的线粒体融合促进线粒体形成更多的PDM,而PDM在肝细胞中具有更高的β氧化能力。重要的是,本研究揭示了一种以前未曾披露的机制,即Mfn2/Atgl-线粒体-LD耦合缓解肝脏LDs积累,这是一种从鱼类到四足动物的保守策略。这项研究为线粒体-LD耦合提供了另一个维度,并为肝脏脂肪变性的治疗干预开辟了新途径。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Redox Biology
BIOCHEMISTRY & MOLECULAR BIOLOGY-
CiteScore
19.90
自引率
3.50%
发文量
318
审稿时长
25 days
期刊介绍:
Redox Biology is the official journal of the Society for Redox Biology and Medicine and the Society for Free Radical Research-Europe. It is also affiliated with the International Society for Free Radical Research (SFRRI). This journal serves as a platform for publishing pioneering research, innovative methods, and comprehensive review articles in the field of redox biology, encompassing both health and disease.
Redox Biology welcomes various forms of contributions, including research articles (short or full communications), methods, mini-reviews, and commentaries. Through its diverse range of published content, Redox Biology aims to foster advancements and insights in the understanding of redox biology and its implications.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


