PTPRZ1 dephosphorylates and stabilizes RNF26 to reduce the efficacy of TKIs and PD-1 blockade in ccRCC
IF 6.9
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
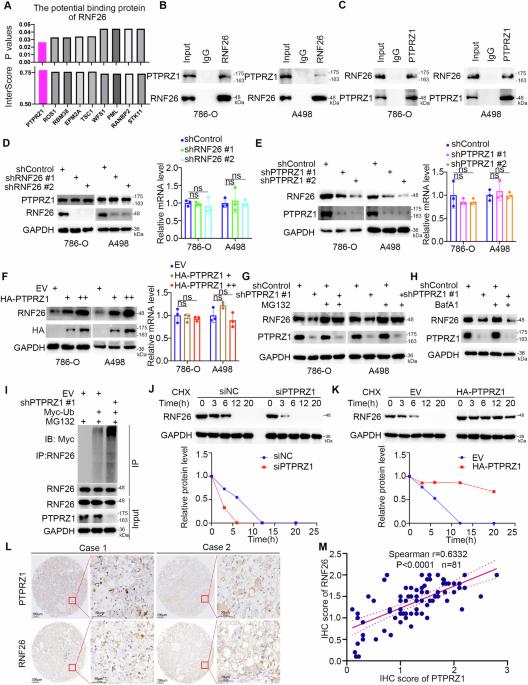
Clear cell renal cell carcinoma (ccRCC), the most common subtype of renal cell carcinoma, often exhibits resistance to tyrosine kinase inhibitors (TKIs) when used as monotherapy. However, the integration of PD-1 blockade with TKIs has significantly improved patient survival, making it a leading therapeutic strategy for ccRCC. Despite these advancements, the efficacy of this combined therapy remains suboptimal, necessitating a deeper understanding of the underlying regulatory mechanisms. Through comprehensive analyses, including mass spectrometry, RNA sequencing, lipidomic profiling, immunohistochemical staining, and ex vivo experiments, we explored the interaction between PTPRZ1 and RNF26 and its impact on ccRCC cell behavior. Our results revealed a unique interaction where PTPRZ1 stabilized RNF26 protein expression by dephosphorylating it at the Y432 site. The modulation of RNF26 levels by PTPRZ1 was found to be mediated through the proteasome pathway. Additionally, PTPRZ1, via its interaction with RNF26, activated the TNF/NF-κB signaling pathway, thereby promoting cell proliferation, angiogenesis, and lipid metabolism in ccRCC cells. Importantly, inhibiting PTPRZ1 enhanced the sensitivity of ccRCC to TKIs and PD-1 blockade, an effect that was attenuated when RNF26 was simultaneously knocked down. These findings highlight the critical role of the PTPRZ1-RNF26 axis in ccRCC and suggest that combining PTPRZ1 inhibitors with current TKIs and PD-1 blockade therapies could significantly improve treatment outcomes for ccRCC patients.
PTPRZ1 可使 RNF26 去磷酸化并使其稳定,从而降低 TKIs 和 PD-1 阻断剂在 ccRCC 中的疗效。
透明细胞肾细胞癌(ccRCC)是肾细胞癌中最常见的亚型,在单药治疗时通常会对酪氨酸激酶抑制剂(TKIs)产生耐药性。然而,将 PD-1 阻断与 TKIs 结合使用可显著提高患者生存率,使其成为 ccRCC 的主要治疗策略。尽管取得了这些进展,但这种联合疗法的疗效仍不理想,因此有必要深入了解其潜在的调控机制。通过质谱分析、RNA测序、脂质组分析、免疫组化染色和体内外实验等综合分析,我们探索了PTPRZ1和RNF26之间的相互作用及其对ccRCC细胞行为的影响。我们的结果发现了一种独特的相互作用,即 PTPRZ1 通过在 Y432 位点使 RNF26 蛋白去磷酸化,从而稳定 RNF26 蛋白的表达。PTPRZ1对RNF26水平的调节是通过蛋白酶体途径介导的。此外,PTPRZ1通过与RNF26相互作用,激活了TNF/NF-κB信号通路,从而促进了ccRCC细胞的增殖、血管生成和脂质代谢。重要的是,抑制 PTPRZ1 会增强 ccRCC 对 TKIs 和 PD-1 阻断的敏感性,而同时敲除 RNF26 会减弱这种效应。这些发现凸显了PTPRZ1-RNF26轴在ccRCC中的关键作用,并表明将PTPRZ1抑制剂与目前的TKIs和PD-1阻断疗法相结合可显著改善ccRCC患者的治疗效果。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Oncogene
医学-生化与分子生物学
CiteScore
15.30
自引率
1.20%
发文量
404
审稿时长
1 months
期刊介绍:
Oncogene is dedicated to advancing our understanding of cancer processes through the publication of exceptional research. The journal seeks to disseminate work that challenges conventional theories and contributes to establishing new paradigms in the etio-pathogenesis, diagnosis, treatment, or prevention of cancers. Emphasis is placed on research shedding light on processes driving metastatic spread and providing crucial insights into cancer biology beyond existing knowledge.
Areas covered include the cellular and molecular biology of cancer, resistance to cancer therapies, and the development of improved approaches to enhance survival. Oncogene spans the spectrum of cancer biology, from fundamental and theoretical work to translational, applied, and clinical research, including early and late Phase clinical trials, particularly those with biologic and translational endpoints.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


