Role of glia in delirium: proposed mechanisms and translational implications
IF 9.6
1区 医学
Q1 BIOCHEMISTRY & MOLECULAR BIOLOGY
引用次数: 0
Abstract
Delirium is a common acute onset neurological syndrome characterised by transient fluctuations in cognition. It affects over 20% of medical inpatients and 50% of those critically ill. Delirium is associated with morbidity and mortality, causes distress to patients and carers, and has significant socioeconomic costs in ageing populations. Despite its clinical significance, the pathophysiology of delirium is understudied, and many underlying cellular mechanisms remain unknown. There are currently no effective pharmacological treatments which directly target underlying disease processes. Although many studies focus on neuronal dysfunction in delirium, glial cells, primarily astrocytes, microglia, and oligodendrocytes, and their associated systems, are increasingly implicated in delirium pathophysiology. In this review, we discuss current evidence which implicates glial cells in delirium, including biomarker studies, post-mortem tissue analyses and pre-clinical models. In particular, we focus on how astrocyte pathology, including aberrant brain energy metabolism and glymphatic dysfunction, reactive microglia, blood-brain barrier impairment, and white matter changes may contribute to the pathogenesis of delirium. We also outline limitations in this body of work and the unique challenges faced in identifying causative mechanisms in delirium. Finally, we discuss how established neuroimaging and single-cell techniques may provide further mechanistic insight at pre-clinical and clinical levels.

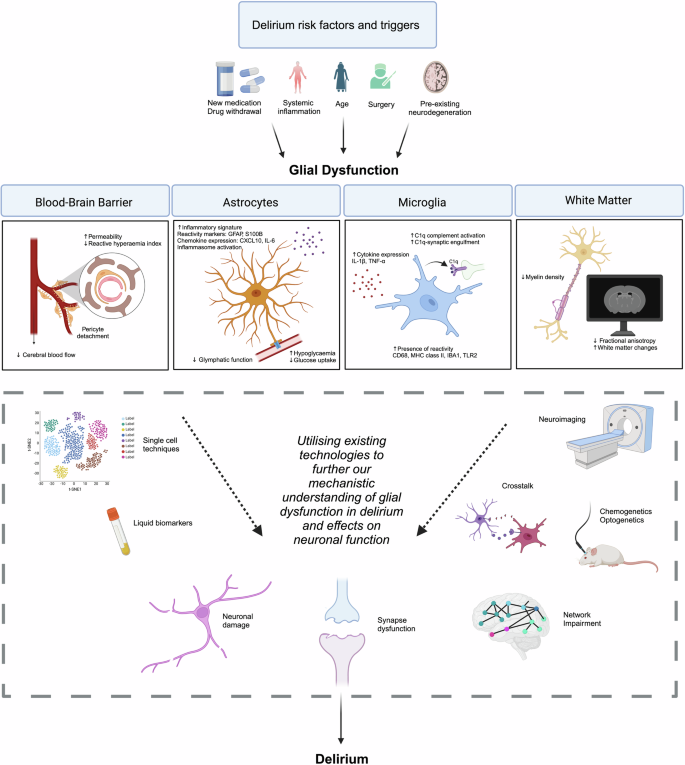
神经胶质细胞在谵妄中的作用:拟议机制和转化意义。
谵妄是一种常见的急性神经系统综合征,其特征是短暂的认知波动。20% 以上的住院病人和 50% 的危重病人都会受到影响。谵妄与发病率和死亡率相关,给患者和护理者带来痛苦,并在老龄化人口中造成巨大的社会经济损失。尽管谵妄具有重要的临床意义,但对其病理生理学的研究却不足,许多潜在的细胞机制仍然未知。目前还没有直接针对潜在疾病过程的有效药物治疗方法。尽管许多研究关注谵妄中的神经元功能障碍,但神经胶质细胞(主要是星形胶质细胞、小胶质细胞和少突胶质细胞)及其相关系统越来越多地与谵妄的病理生理学有关。在这篇综述中,我们将讨论目前证明神经胶质细胞与谵妄有关的证据,包括生物标志物研究、死后组织分析和临床前模型。我们特别关注星形胶质细胞的病理变化,包括异常的脑能量代谢和脑功能障碍、反应性小胶质细胞、血脑屏障损伤和白质变化如何可能导致谵妄的发病机制。我们还概述了这方面研究的局限性,以及在确定谵妄致病机制时所面临的独特挑战。最后,我们讨论了成熟的神经成像和单细胞技术如何在临床前和临床层面提供进一步的机理见解。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Psychiatry
医学-精神病学
CiteScore
20.50
自引率
4.50%
发文量
459
审稿时长
4-8 weeks
期刊介绍:
Molecular Psychiatry focuses on publishing research that aims to uncover the biological mechanisms behind psychiatric disorders and their treatment. The journal emphasizes studies that bridge pre-clinical and clinical research, covering cellular, molecular, integrative, clinical, imaging, and psychopharmacology levels.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


