TGF-β antagonism synergizes with PPARγ agonism to reduce fibrosis and enhance beige adipogenesis
IF 7
2区 医学
Q1 ENDOCRINOLOGY & METABOLISM
引用次数: 0
Abstract
Objectives
Adipose tissue depots vary markedly in their ability to store and metabolize triglycerides, undergo beige adipogenesis and susceptibility to metabolic disease. The molecular mechanisms that underlie such heterogeneity are not entirely clear. Previously, we showed that TGF-β signaling suppresses beige adipogenesis via repressing the recruitment of dedicated beige progenitors. Here, we find that TGF-β signals dynamically regulate the balance between adipose tissue fibrosis and beige adipogenesis.
Methods
We investigated adipose tissue depot-specific differences in activation of TGF-β signaling in response to dietary challenge. RNA-seq and fluorescence activated cell sorting was performed to identify and characterize cells responding to changes in TGF-β signaling status. Mouse models, pharmacological strategies and human adipose tissue analyses were performed to further define the influence of TGF-β signaling on fibrosis and functional beige adipogenesis.
Results
Elevated basal and high-fat diet inducible activation of TGF-β/Smad3 signaling was observed in the visceral adipose tissue depot. Activation of TGF-β/Smad3 signaling was associated with increased adipose tissue fibrosis. RNA-seq combined with fluorescence-activated cell sorting of stromal vascular fraction of epididymal white adipose tissue depot resulted in identification of TGF-β/Smad3 regulated ITGA5+ fibrogenic progenitors. TGF-β/Smad3 signal inhibition, genetically or pharmacologically, reduced fibrosis and increased functional beige adipogenesis. TGF-β/Smad3 antagonized the beneficial effects of PPARγ whereas TGF-β receptor 1 inhibition synergized with actions of rosiglitazone, a PPARγ agonist, to dampen fibrosis and promote beige adipogenesis. Positive correlation between TGF-β activation and ITGA5 was observed in human adipose tissue, with visceral adipose tissue depots exhibiting higher fibrosis potential than subcutaneous or brown adipose tissue depots.
Conclusions
Basal and high-fat diet inducible activation of TGF-β underlies the heterogeneity of adipose tissue depots. TGF-β/Smad3 activation promotes adipose tissue fibrosis and suppresses beige progenitors. Together, these dual mechanisms preclude functional beige adipogenesis. Controlled inhibition of TβRI signaling and concomitant PPARγ stimulation can suppress adipose tissue fibrosis and promote beige adipogenesis to improve metabolism.
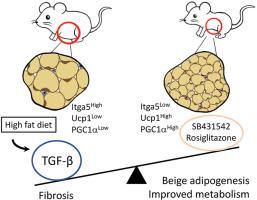
TGF-β 拮抗作用与 PPARγ 激动作用协同作用,可减少纤维化并促进米色脂肪生成。
目的:脂肪组织库在储存和代谢甘油三酯的能力、米色脂肪生成以及对代谢性疾病的易感性方面存在明显差异。导致这种异质性的分子机制尚不完全清楚。此前,我们发现 TGF-β 信号通过抑制专用米色祖细胞的招募来抑制米色脂肪的生成。在此,我们发现 TGF-β 信号可动态调节脂肪组织纤维化和米色脂肪生成之间的平衡:方法:我们研究了TGF-β信号激活对饮食挑战反应的脂肪组织特异性差异。通过RNA-seq和荧光激活细胞分选技术,我们识别了响应TGF-β信号状态变化的细胞并确定了其特征。通过小鼠模型、药理策略和人体脂肪组织分析,进一步确定了TGF-β信号转导对纤维化和功能性米色脂肪生成的影响:结果:在内脏脂肪组织库中观察到TGF-β/Smad3信号的基础激活和高脂饮食诱导激活升高。TGF-β/Smad3信号的激活与脂肪组织纤维化的增加有关。RNA-seq结合荧光激活细胞分选技术对附睾白色脂肪组织基质血管部分进行研究,结果发现了受TGF-β/Smad3调控的ITGA5+纤维化祖细胞。通过基因或药物抑制 TGF-β/Smad3 信号可减少纤维化并增加功能性米色脂肪生成。TGF-β/Smad3拮抗了PPARγ的有益作用,而TGF-β受体1抑制与PPARγ激动剂罗格列酮协同作用,抑制纤维化并促进米色脂肪生成。在人体脂肪组织中观察到了TGF-β激活与ITGA5之间的正相关性,内脏脂肪组织库比皮下或棕色脂肪组织库表现出更高的纤维化潜力:结论:基础和高脂饮食诱导的 TGF-β 激活是脂肪组织储层异质性的基础。TGF-β/Smad3活化可促进脂肪组织纤维化并抑制米色祖细胞。这些双重机制共同排除了功能性米色脂肪生成。有控制地抑制 TβR1 信号传导并同时刺激 PPARγ 可抑制脂肪组织纤维化并促进米色脂肪生成,从而改善新陈代谢。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Molecular Metabolism
ENDOCRINOLOGY & METABOLISM-
CiteScore
14.50
自引率
2.50%
发文量
219
审稿时长
43 days
期刊介绍:
Molecular Metabolism is a leading journal dedicated to sharing groundbreaking discoveries in the field of energy homeostasis and the underlying factors of metabolic disorders. These disorders include obesity, diabetes, cardiovascular disease, and cancer. Our journal focuses on publishing research driven by hypotheses and conducted to the highest standards, aiming to provide a mechanistic understanding of energy homeostasis-related behavior, physiology, and dysfunction.
We promote interdisciplinary science, covering a broad range of approaches from molecules to humans throughout the lifespan. Our goal is to contribute to transformative research in metabolism, which has the potential to revolutionize the field. By enabling progress in the prognosis, prevention, and ultimately the cure of metabolic disorders and their long-term complications, our journal seeks to better the future of health and well-being.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


