Gastrointestinal pathophysiology in long COVID: Exploring roles of microbiota dysbiosis and serotonin dysregulation in post-infectious bowel symptoms
IF 5.1
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
The emergence of severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) triggered an unprecedented public health crisis known as the coronavirus disease 2019 (COVID-19) pandemic. Gastrointestinal (GI) symptoms develop in patients during acute infection and persist after recovery from airway distress in a chronic form of the disease (long COVID). A high incidence of irritable bowel syndrome (IBS) manifested by severe abdominal pain and defecation pattern changes is reported in COVID patients. Although COVID is primarily considered a respiratory disease, fecal shedding of SARS-CoV-2 antigens positively correlates with bowel symptoms. Active viral infection in the GI tract was identified by human intestinal organoid studies showing SARS-CoV-2 replication in gut epithelial cells. In this review, we highlight the key findings in post-COVID bowel symptoms and explore possible mechanisms underlying the pathophysiology of the illness. These mechanisms include mucosal inflammation, gut barrier dysfunction, and microbiota dysbiosis during viral infection. Viral shedding through the GI route may be the primary factor causing the alteration of the microbiome ecosystem, particularly the virome. Recent evidence in experimental models suggested that microbiome dysbiosis could be further aggravated by epithelial barrier damage and immune activation. Moreover, altered microbiota composition has been associated with dysregulated serotonin pathways, resulting in intestinal nerve hypersensitivity. These mechanisms may explain the development of post-infectious IBS-like symptoms in long COVID. Understanding how coronavirus infection affects gut pathophysiology, including microbiome changes, would benefit the therapeutic advancement for managing post-infectious bowel symptoms.
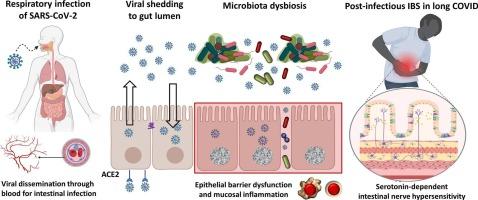
长期 COVID 的胃肠道病理生理学:探索微生物群失调和血清素失调在感染后肠道症状中的作用。
严重急性呼吸系统综合征冠状病毒 2(SARS-CoV-2)的出现引发了一场前所未有的公共卫生危机,即冠状病毒病 2019(COVID-19)大流行。在急性感染期间,患者会出现胃肠道(GI)症状,而在慢性病(长COVID)中,患者在从气道窘迫中恢复后仍会出现胃肠道(GI)症状。据报道,COVID 患者中肠易激综合征(IBS)的发病率很高,表现为剧烈腹痛和排便模式改变。尽管 COVID 主要被认为是一种呼吸道疾病,但粪便中的 SARS-CoV-2 抗原脱落与肠道症状呈正相关。人体肠道类器官研究显示,SARS-CoV-2 在肠道上皮细胞中复制,从而确定了病毒在消化道中的活跃感染。在本综述中,我们将重点介绍有关后 COVID 肠道症状的主要发现,并探讨该疾病病理生理学的可能机制。这些机制包括病毒感染期间的粘膜炎症、肠道屏障功能障碍和微生物群失调。病毒通过消化道途径脱落可能是导致微生物群生态系统,特别是病毒群发生改变的主要因素。实验模型的最新证据表明,上皮屏障损伤和免疫激活会进一步加剧微生物群失调。此外,微生物群组成的改变与血清素通路失调有关,从而导致肠道神经过敏。这些机制可能解释了长COVID感染后会出现类似肠易激综合征的症状。了解冠状病毒感染如何影响肠道病理生理学,包括微生物群的变化,将有利于治疗感染后肠道症状。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


