Association between hepatocyte TM4SF5 expression and gut microbiome dysbiosis during non-alcoholic fatty liver disease development
IF 5.2
2区 医学
Q1 MEDICINE, RESEARCH & EXPERIMENTAL
引用次数: 0
Abstract
Gut microbiome dysbiosis is involved in non-alcoholic fatty liver disease (NAFLD) development. Hepatic transmembrane 4 L six family member 5 (TM4SF5) overexpression promotes NAFLD. However, how gut microbiota are associated with TM4SF5-mediated NAFLD remains unexplored. We analyzed the gut microbiome using feces from hepatocyte-specific TM4SF5-overexpressing transgenic (Alb-TGTm4sf5-Flag, TG) or Tm4sf5−/− knock-out (KO) mice fed a normal chow diet (NCD), high-fat diet (HFD) for 2 weeks (HFD2W), or methionine-choline-deficient diet (MCD) for 4 weeks to investigate associations among Tm4sf5 expression, diet, and the gut microbiome. TG-NCD mice showed a higher Firmicutes-to-Bacteroidetes (F/B) ratio, with less enrichment of Akkermansia muciniphila and Lactobacillus reuteri. NASH-related microbiomes in feces were more abundant in TG-HFD2w mice than in KO-HFD2w mice. Further, TG-MCD showed a higher F/B ratio than TG-NCD or KO mice, with decreases or increases in microbiomes beneficial or detrimental to the liver, respectively. Such effects in TG-MCD animals were correlated with functional pathways producing short-chain fatty acids (SCFAs). Furthermore, potential functional pathways of the gut microbiome were metabolically parallel to NAFLD features in TG-MCD mice. These results suggest that hepatocyte Tm4sf5 supports gut microbiome dysbiosis and metabolic activity, leading to SCFA production and hepatic inflammation during NAFLD development.
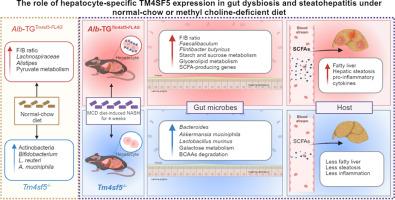
非酒精性脂肪肝发展过程中肝细胞 TM4SF5 表达与肠道微生物群失调之间的关系
肠道微生物群失调与非酒精性脂肪肝(NAFLD)的发生有关。肝脏跨膜 4 L 6 家族成员 5(TM4SF5)过表达会促进非酒精性脂肪肝。然而,肠道微生物群如何与 TM4SF5 介导的非酒精性脂肪肝相关联仍有待探索。我们利用肝细胞特异性 TM4SF5 表达过高的转基因(Alb-TGTm4sf5-Flag,TG)或 Tm4sf5-/- 基因敲除(KO)小鼠的粪便分析了肠道微生物群,这些小鼠分别以正常饲料(NCD)、高脂饲料(HFD)喂养 2 周(HFD2W)或蛋氨酸-胆碱缺乏饲料(MCD)喂养 4 周,以研究 Tm4sf5 表达、饮食和肠道微生物群之间的关系。TG-NCD小鼠显示出更高的固着菌-类杆菌(F/B)比率,Akkermansia muciniphila和Lactobacillus reuteri的富集程度较低。与 KO-HFD2w 小鼠相比,TG-HFD2w 小鼠粪便中的 NASH 相关微生物群更为丰富。此外,与 TG-NCD 或 KO 小鼠相比,TG-MCD 表现出更高的 F/B 比率,对肝脏有益或有害的微生物群分别减少或增加。TG-MCD动物的这种影响与产生短链脂肪酸(SCFAs)的功能途径相关。此外,肠道微生物组的潜在功能途径与 TG-MCD 小鼠非酒精性脂肪肝的代谢特征平行。这些结果表明,肝细胞 Tm4sf5 支持肠道微生物组的菌群失调和代谢活动,导致非酒精性脂肪肝发展过程中产生 SCFA 和肝脏炎症。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Life sciences
医学-药学
CiteScore
12.20
自引率
1.60%
发文量
841
审稿时长
6 months
期刊介绍:
Life Sciences is an international journal publishing articles that emphasize the molecular, cellular, and functional basis of therapy. The journal emphasizes the understanding of mechanism that is relevant to all aspects of human disease and translation to patients. All articles are rigorously reviewed.
The Journal favors publication of full-length papers where modern scientific technologies are used to explain molecular, cellular and physiological mechanisms. Articles that merely report observations are rarely accepted. Recommendations from the Declaration of Helsinki or NIH guidelines for care and use of laboratory animals must be adhered to. Articles should be written at a level accessible to readers who are non-specialists in the topic of the article themselves, but who are interested in the research. The Journal welcomes reviews on topics of wide interest to investigators in the life sciences. We particularly encourage submission of brief, focused reviews containing high-quality artwork and require the use of mechanistic summary diagrams.
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


