Development of an ELISA for an effective potency determination of recombinant rabies human monoclonal antibody
IF 1.6
4区 医学
Q4 BIOCHEMICAL RESEARCH METHODS
引用次数: 0
Abstract
Rapid Fluorescence Focus Inhibition Test (RFFIT) is the most widely used cell-based assay to measure the potency of recombinant human rabies monoclonal antibodies. Nonetheless, RFFIT assay is time-consuming and it requires well-equipped biosafety level 2 facility, virulent live rabies virus cultures, permissive cell lines, and well-trained manpower. Therefore, the development of alternative methods to the RFFIT has been encouraged by the World Health Organization (WHO) expert working groups to overcome these barriers.
An In-vitro ELISA test has been developed as an alternative to the RFFIT assay, for quantifying the rabies monoclonal antibody (mAb) potency using inactivated rabies virus vaccine (Rabivax-S). It is based on the specific interaction between the antigen and the antibody, that induces neutralizing antibody response to rabies virus.
The ELISA was validated involving accuracy and precision within 20 % coefficient of variance. The validation has been done by 4PL standard curve with linearity r2 ˃ 0.98 and LLOQ of 0.3 μg/mL indicating high assay sensitivity. The specificity of the assay was ascertained by challenging with another homologous non-rabies humanized mAb, which does not show binding with the rabies virus.
The indirect ELISA developed here, is precise, robust, and accurate to quantitate the potency of rabies monoclonal antibody. It is highly sensitive and has a broad range of detection. It is easy to perform, and it has a short turnaround time (results available in few hours). Furthermore, it is cost effective and can be performed with low-cost resource setting, as there is no requirement of handling the live cells and live virus and also BSL-2 Facility.
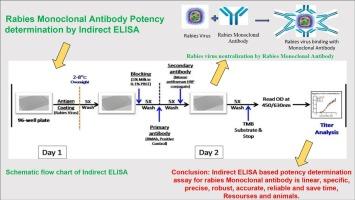
开发一种有效测定重组狂犬病人类单克隆抗体效价的酶联免疫吸附试验。
快速荧光聚焦抑制试验(RFFIT)是最广泛使用的基于细胞的检测方法,用于测量重组人狂犬病单克隆抗体的效力。然而,快速荧光聚焦抑制试验非常耗时,而且需要设备齐全的 2 级生物安全设施、活狂犬病毒毒力培养物、许可细胞系和训练有素的人员。因此,世界卫生组织(WHO)专家工作组鼓励开发 RFFIT 的替代方法,以克服这些障碍。目前已开发出一种体外 ELISA 检测法,作为 RFFIT 检测法的替代方法,利用灭活狂犬病毒疫苗 (Rabivax-S) 量化狂犬病单克隆抗体 (mAb) 的效力。该方法基于抗原与抗体之间的特异性相互作用,可诱导狂犬病病毒中和抗体反应。酶联免疫吸附试验的准确度和精密度在 20% 的方差系数范围内。通过 4PL 标准曲线进行了验证,线性关系 r2 ˃ o.98,LLOQ 为 0.3 μg/mL,表明检测灵敏度很高。通过与另一种同源的非狂犬病人源化 mAb 进行比对,确定了该检测方法的特异性。在此开发的间接 ELISA 能精确、稳健、准确地量化狂犬病单克隆抗体的效价。它灵敏度高,检测范围广。它易于操作,周转时间短(几小时内即可得到结果)。此外,由于不需要处理活细胞和活病毒,也不需要 BSL-2 设施,因此成本效益高,可在低成本资源设置下进行。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊
CiteScore
4.10
自引率
0.00%
发文量
120
审稿时长
3 months
期刊介绍:
The Journal of Immunological Methods is devoted to covering techniques for: (1) Quantitating and detecting antibodies and/or antigens. (2) Purifying immunoglobulins, lymphokines and other molecules of the immune system. (3) Isolating antigens and other substances important in immunological processes. (4) Labelling antigens and antibodies. (5) Localizing antigens and/or antibodies in tissues and cells. (6) Detecting, and fractionating immunocompetent cells. (7) Assaying for cellular immunity. (8) Documenting cell-cell interactions. (9) Initiating immunity and unresponsiveness. (10) Transplanting tissues. (11) Studying items closely related to immunity such as complement, reticuloendothelial system and others. (12) Molecular techniques for studying immune cells and their receptors. (13) Imaging of the immune system. (14) Methods for production or their fragments in eukaryotic and prokaryotic cells.
In addition the journal will publish articles on novel methods for analysing the organization, structure and expression of genes for immunologically important molecules such as immunoglobulins, T cell receptors and accessory molecules involved in antigen recognition, processing and presentation. Submitted full length manuscripts should describe new methods of broad applicability to immunology and not simply the application of an established method to a particular substance - although papers describing such applications may be considered for publication as a short Technical Note. Review articles will also be published by the Journal of Immunological Methods. In general these manuscripts are by solicitation however anyone interested in submitting a review can contact the Reviews Editor and provide an outline of the proposed review.

 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


