TREM1 induces microglial ferroptosis through the PERK pathway in diabetic-associated cognitive impairment
IF 4.6
2区 医学
Q1 NEUROSCIENCES
引用次数: 0
Abstract
Ferroptosis is involved in neurodegenerative disorders including diabetes-associated cognitive impairment (DACI). As central immune cells, microglia have strong siderophilic properties. However, the role of iron deposition in microglia and the underlying regulatory mechanism remains unclear in DACI. Here, we established high glucose (HG) model in BV2/HMC3 cells and diabetes model in C57BL/6 J mice with HFD and STZ. Transmission Electron Microscopy, Western blot, assay kits of Fe2+, GSH/GSSG, MDA and ROS were carried out in vitro. Prussian blue staining, Western blot and immunofluorescence were implemented in vivo. Y-maze and novel object recognition were performed to assess cognitive performance. LP17 was used to inhibit TREM1 (triggering receptor expressed on myeloid cells 1) specifically in vivo and vitro. We found excessively deposited iron and significant reduction in antioxidants in hippocampal microglia of mice with DACI, concomitant with increased TREM1 (a microglia-specific inflammatory amplifier). Furthermore, LP17 (TREM1 specific inhibitor) ameliorated cognitive impairment caused by HFD/STZ through relieving iron accumulation and antioxidant inactivation. In vitro, ferroptosis was induced by HG in mice microglia-BV2 and human microglia-HMC3 cells, which could be blocked by a ferroptosis inhibitor-Fer-1 and LP17. Moreover, PERK pathway of endoplasmic reticulum stress was activated by HG, and then reversed by PERK inhibitor GSK2606414 and LP17 followed by improved ferroptosis in HG-cultured BV2. In summary, our results indicated that TREM1 effectively aggravates T2DM-associated microglial iron accumulation through the PERK pathway of ERS, which contributes to antioxidant inactivation and lipid peroxidation, eventually, massively boosted ROS result in microglial ferroptosis. The mechanism elucidation in our study may shed light on targeted therapy of DACI.
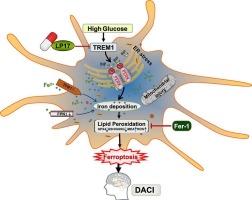
在糖尿病相关认知障碍中,TREM1 通过 PERK 通路诱导小胶质细胞铁突变。
铁蛋白沉积与神经退行性疾病有关,包括糖尿病相关认知障碍(DACI)。作为中枢免疫细胞,小胶质细胞具有很强的嗜铁特性。然而,铁沉积在小胶质细胞中的作用以及在 DACI 中的潜在调控机制仍不清楚。在此,我们在 BV2/HMC3 细胞中建立了高糖(HG)模型,并在 C57BL/6 J 小鼠中建立了 HFD 和 STZ 糖尿病模型。在体外进行了透射电子显微镜、Western blot、Fe2+、GSH/GSSG、MDA 和 ROS 检测。在体内进行了普鲁士蓝染色、Western印迹和免疫荧光。进行了Y-迷宫和新物体识别以评估认知能力。LP17 被用于抑制体内和体外的 TREM1(髓样细胞上表达的触发受体 1)。我们发现,在DACI小鼠的海马小胶质细胞中,铁沉积过多,抗氧化剂显著减少,同时TREM1(一种小胶质细胞特异性炎症放大器)增加。此外,LP17(TREM1特异性抑制剂)通过缓解铁积累和抗氧化剂失活,改善了HFD/STZ导致的认知障碍。在体外,HG诱导小鼠小胶质细胞-BV2和人小胶质细胞-HMC3细胞发生铁变态反应,铁变态反应抑制剂-Fer-1和LP17可阻断这种反应。此外,内质网应激的PERK通路被HG激活,然后被PERK抑制剂GSK2606414和LP17逆转,随后HG培养的BV2的铁突变得到改善。综上所述,我们的研究结果表明,TREM1通过ERS的PERK通路有效地加重了T2DM相关的小胶质细胞铁蓄积,从而导致抗氧化剂失活和脂质过氧化,最终,ROS的大量增加导致小胶质细胞铁嗜酸性化。我们的研究对这一机制的阐明或许能为DACI的靶向治疗提供启示。
本文章由计算机程序翻译,如有差异,请以英文原文为准。
求助全文
约1分钟内获得全文
求助全文
来源期刊

Experimental Neurology
医学-神经科学
CiteScore
10.10
自引率
3.80%
发文量
258
审稿时长
42 days
期刊介绍:
Experimental Neurology, a Journal of Neuroscience Research, publishes original research in neuroscience with a particular emphasis on novel findings in neural development, regeneration, plasticity and transplantation. The journal has focused on research concerning basic mechanisms underlying neurological disorders.
文献相关原料
公司名称
产品信息
上海源叶
DCFH-DA
 求助内容:
求助内容: 应助结果提醒方式:
应助结果提醒方式:


